Blue electroluminescent compounds and application thereof in organic electroluminescent devices
A technology of light-emitting compounds and electroluminescent materials, which is applied in the fields of electric solid devices, organic chemistry, light-emitting materials, etc., can solve the problems of the scarcity of blue organic light-emitting materials, and achieves favorable transmission balance, simple preparation, and easy preparation and operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
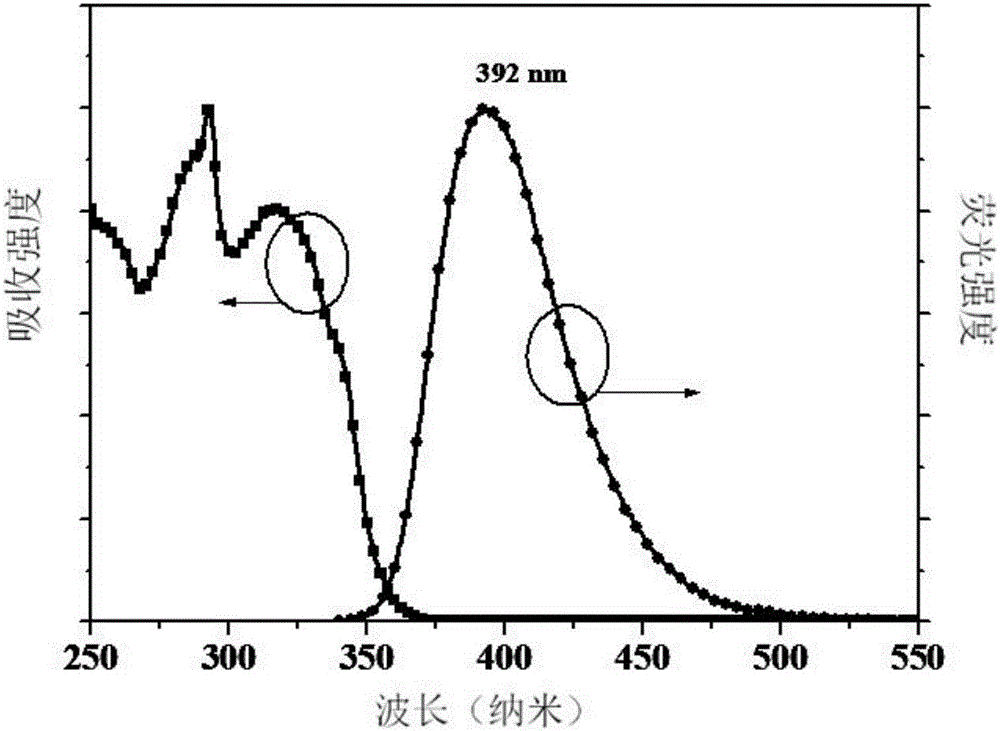
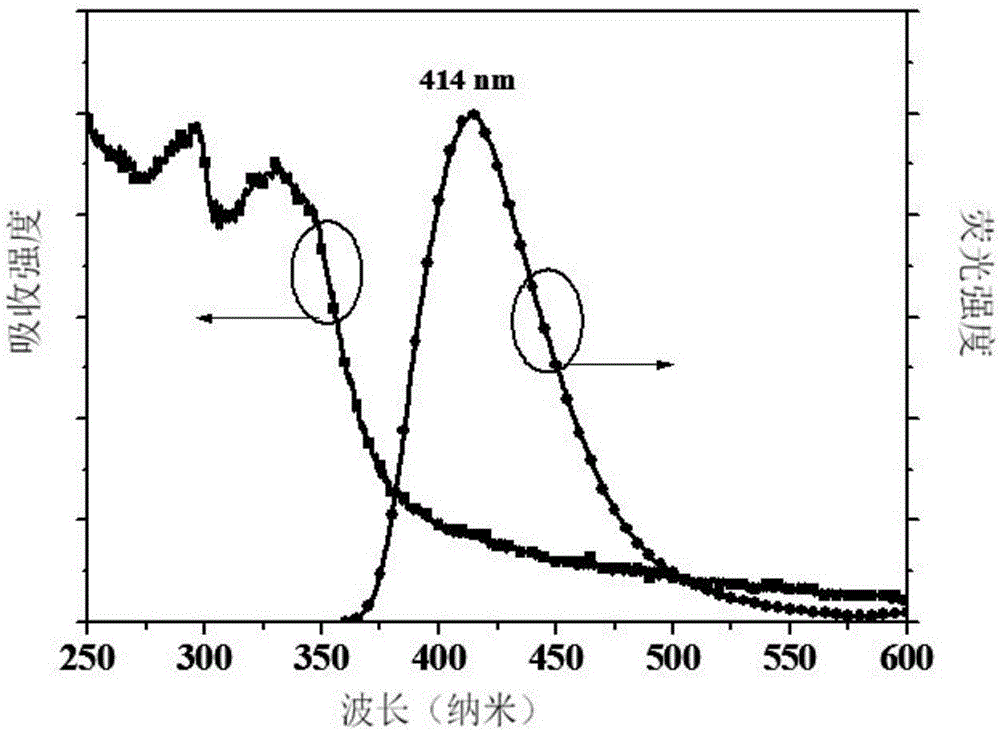
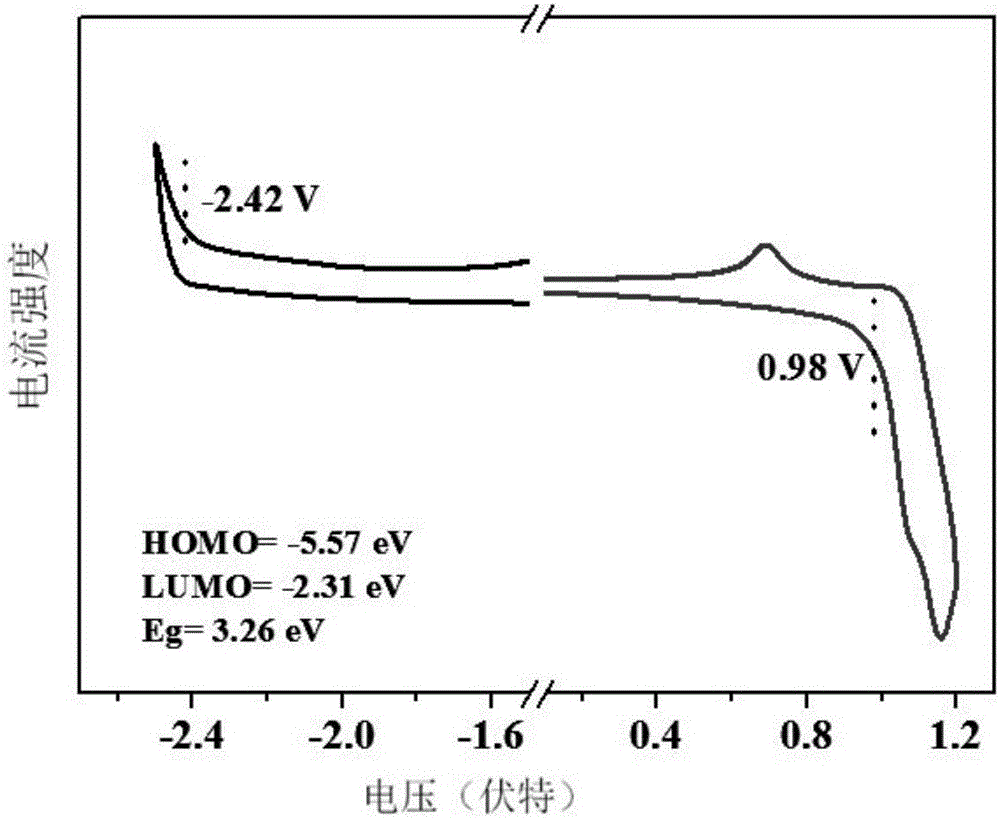
[0037] The near-ultraviolet organic electroluminescent compound prepared in this example is 9-(4'-(4,5-diphenyl-4H-1,2,4-triazole)-1,1'-diphenyl)- 9H-carbazole, whose molecular formula is C 38 h 26 N 4 , the structural formula is:
[0038]
[0039] Concrete preparation steps are as follows:
[0040]
[0041] 1. Preparation of precursor 3-(4-bromophenyl)-4,5-diphenyl-4H-1,2,4-triazole
[0042] Mix 5.59g of aniline into 10mL of 1,2-o-dichlorobenzene, add 7.5mL of phosphorus trichloride into it with a syringe, and react at 100°C for 1h. Afterwards, 3.19 g of N-benzoyl-N′-aromatic hydrazide was added to the above system, and reacted at 200° C. for 24 h.
[0043] After the reaction was cooled to room temperature, the reaction system was poured into deionized water, and a yellow solid appeared, which was washed with water and filtered. The filtered solid was dried in an oven. Afterwards, dichloromethane / ethyl acetate was used as eluent, and column chromatography gave a ...
Embodiment 2
[0051] The organic electroluminescent compound 9-(4'-(4,5-diphenyl-4H-1,2,4-triazole)-1,1'-diphenyl)-9H-carbazole was used as the light-emitting layer To prepare a non-doped electroluminescent device, the specific steps are as follows:
[0052] 1. Clean the ITO glass in acetone, cleaning agent, deionized water and isopropanol in a cycle of ultrasonic cleaning, dry at 120°C, and expose to UV-ozone atmosphere for 20 minutes.
[0053] 2. Under a certain degree of vacuum, the hole injection layer, the hole transport layer, the electron blocking layer, the emitting layer (Emitting-layer EML), the electron transport layer, the electron injection layer and the cathode are sequentially evaporated at a certain rate. The device structure is ITO / MoO 3 (8nm) / NPB(80nm) / TCTA(10nm) / EML(20nm) / TPBi(40nm) / LiF(1nm) / Al(100nm) Figure 4 . Among them, the structural formulas of NPB, TCTA, and TPBi are as follows:
[0054]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com