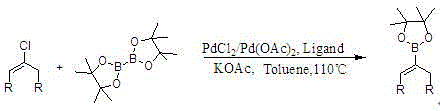Synthesis method of cycloalkene-1-boronic acid pinacol ester
A technology of pinacol ester and synthesis method, which is applied in the field of organic chemical synthesis, can solve the problems of difficult purification, cumbersome operation, and high cost of raw materials, and achieve the effects of cheap and easy-to-obtain raw materials, reduced process difficulty, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Taking the preparation of cyclohexene-1-boronic acid pinacol ester as an example, taking cyclohexanone as raw material, solvent A For n-heptane, solvent B is tetrahydrofuran, solvent C to ethyl acetate
[0030] The preparation of the first step raw material 1-chlorocyclohexene:
[0031] Add 320.3g (1.556 mol, 1.05eq) of phosphorus pentachloride and 801 g of n-heptane into a 2L four-necked flask equipped with a magnetic stirrer, a thermometer, a condenser tube and a tail gas lye absorption device, raise the temperature to 72°C, drop Add 150.2g (1.533mol, 1eq) cyclohexanone, drop it for about 2 hours, continue to react for half an hour, after cooling down to 0°C, add 0.51kg of 6mol / L sodium hydroxide solution dropwise, stir for 1 hour, and separate the upper layer The organic layer was washed once with 100 g of saturated sodium bicarbonate to obtain 757.1 g of 1-chlorocyclohexene solution, with an internal standard yield of 82.2%;
[0032] The preparation of the secon...
Embodiment 2
[0037] Taking the preparation of cyclopentene-1-boronic acid pinacol ester as an example, taking cyclopentanone as raw material, solvent A For n-hexane, solvent B is tetrahydrofuran, solvent C for toluene
[0038] The preparation of the first step raw material 1-chlorocyclopentene:
[0039] Add 374.2 (1.818 mol, 1.05) phosphorus pentachloride and 863 g of n-heptane to a 2L four-neck flask equipped with a magnetic stirrer, thermometer, condenser tube and tail gas alkali absorption device, raise the temperature to 70 ° C, and add dropwise 151.4 g (1.803mol, 1eq) cyclopentanone, drop it in about 2 hours, continue to react for half an hour, after cooling down to 0°C, add 0.69kg of 6mol / L sodium hydroxide solution dropwise, stir for one hour, and separate the upper organic layer , the organic layer was washed once with saturated sodium bicarbonate to obtain 799 g of n-hexane solution of 1-chlorocyclopentene, and the internal standard yield was 81.1%;
[0040]The preparation of ...
Embodiment 3
[0045] Take the preparation of 3,3,5,5-tetramethyl-cyclohexene-1-boronic acid pinacol ester as an example, with 3,3,5,5-tetramethylcyclohexanone as raw material, solvent A For n-heptane, solvent B is tetrahydrofuran, solvent C MTBE
[0046] Preparation of the first step raw material 3,3,5,5-tetramethyl-1-chlorocyclohexene:
[0047] Add 191.5 (0.930 mol, 1.05) phosphorus pentachloride and 663 g of n-heptane to a 2L four-necked flask equipped with a magnetic stirrer, thermometer, condenser tube and tail gas alkali absorption device, raise the temperature to 75 ° C, and drop 136.4 g (0.886mol, 1eq) 3,3,5,5-tetramethylcyclohexanone, after about 2 hours, continue to react for half an hour, after cooling down to 0°C, add dropwise 6mol / L sodium hydroxide solution 0.43 kg, stirred for one hour, separated the upper organic layer, and washed the organic layer once with saturated sodium bicarbonate to obtain 659 g of n-heptane solution of 3,3,5,5-tetramethyl-1-chlorocyclohexene, inter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com