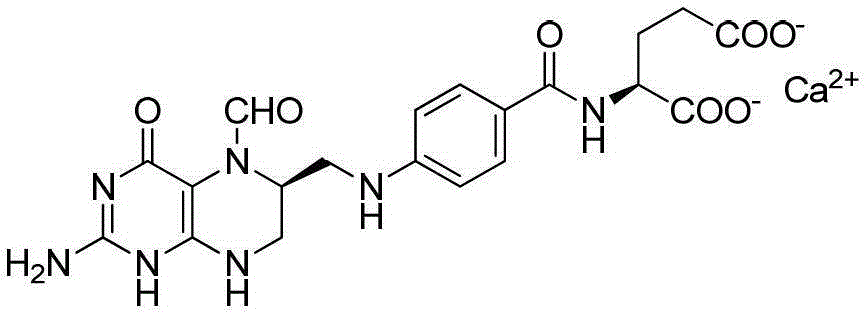
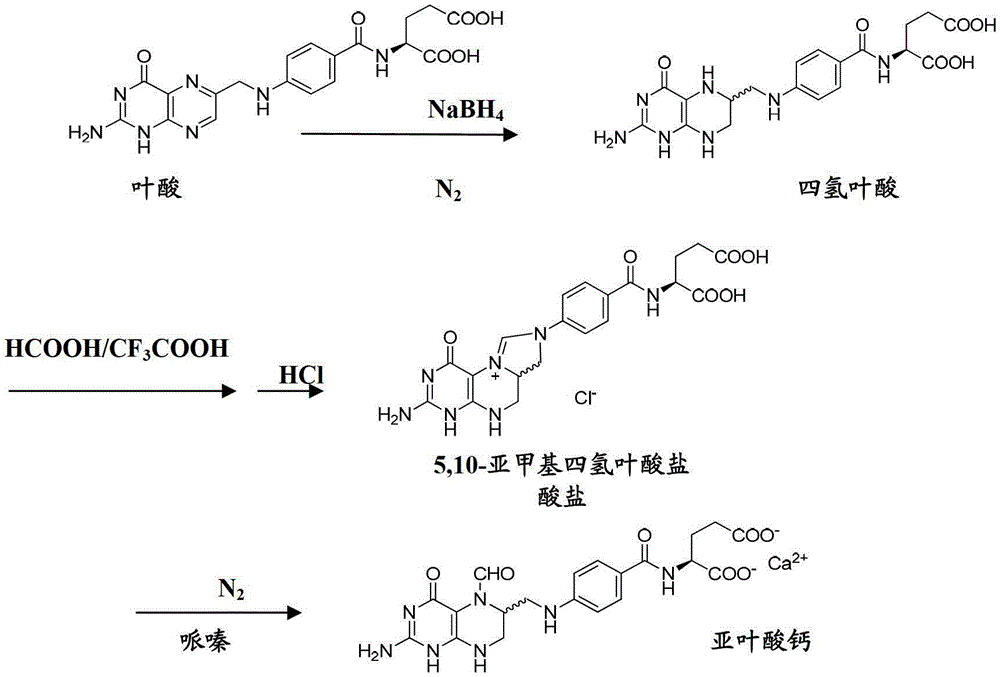
A method for preparing high-purity calcium levofolinate
A technology of calcium levofolinate and methylenetetrahydrofolate, which is applied in skin diseases, bone diseases, antipyretics, etc., can solve the problems that are difficult to remove, difficult to achieve in industrial production conditions, and heating temperature affects the quality of intermediates and final products and other problems, to achieve the effect of reducing requirements, facilitating large-scale operation, and avoiding corrosive reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] 1) Weigh 300g of folic acid, add 2.7L of distilled water, and stir; adjust the pH value of the reaction system to 8.0 with NaOH solution, and inject N 2 For protection, add 200g of NaBH 4 solid, and adjust the pH value to 3.0 with hydrochloric acid, precipitate tetrahydrofolic acid solid, filter; add the filtered solid to 300mL formic acid, after stirring and dissolving, add 6mL of trifluoroacetic acid as a catalyst, at 10°C-30°C Place it at room temperature for 14 hours; then add 75mL, 6mol / L hydrochloric acid to the system to precipitate the formylation intermediate - 5,10-methylenetetrahydrofolate hydrochloride;
[0066] 2) Redissolve 150g of the precipitated 5,10-methylenetetrahydrofolate hydrochloride in 300mL of formic acid, stir and dissolve, then add 75mL of 6mol / L hydrochloric acid, at a temperature of 10°C-30°C (room temperature), the refined product of 5,10-methylenetetrahydrofolate hydrochloride was precipitated, and the detected specific rotation was +24°;...
Embodiment 2
[0070] 1) Weigh 1.0kg of folic acid, add 9.0L of distilled water, and stir; use NaOH solution to adjust the pH value of the reaction system to 8.0, and inject N 2 For protection, add 1.0kgNaBH 4solid, and adjust the pH value to 3.0 with hydrochloric acid, precipitate tetrahydrofolic acid solid, filter; add the filtered solid to 5L formic acid, stir and dissolve, add 200mL of trifluoroacetic acid as a catalyst, at 10°C-30°C Place it at room temperature for 24 hours; then add 12.5 L, 0.5 mol / L hydrochloric acid to the system to precipitate the formylation intermediate - 5,10-methylenetetrahydrofolate hydrochloride;
[0071] 2) Redissolve 300g of the precipitated 5,10-methylenetetrahydrofolate hydrochloride in 1.5L of formic acid, stir to dissolve, add 3.75L, 1mol / L hydrochloric acid, at a temperature of 10°C-30°C (room temperature), the refined product of 5,10-methylenetetrahydrofolate hydrochloride was precipitated, and the detected specific rotation was +18°;
[0072] 3) Add...
Embodiment 3
[0075] 1) Weigh 2.5kg of folic acid, add 25L of distilled water, and stir; use NaOH solution to adjust the pH value of the reaction system to 7.5, and inject N 2 For protection, add 2.5kg of NaBH 4 solid, and adjust the pH value to 3.0 with hydrochloric acid, precipitate tetrahydrofolic acid solid, filter; add the filtered solid to 10L formic acid, stir and dissolve, add 200mL trifluoroacetic acid as a catalyst; After standing at room temperature for 10 hours, add 7.5L of 2mol / L hydrochloric acid to formic acid to precipitate the formylation intermediate - 5,10-methylenetetrahydrofolate hydrochloride;
[0076] 2) Redissolve 700g of the precipitated 5,10-methylenetetrahydrofolate hydrochloride in 2.1L formic acid, stir and dissolve, then add 2.1L, 2mol / L hydrochloric acid, Placed at room temperature, the refined product of 5,10-methylenetetrahydrofolate hydrochloride was precipitated, and the detected specific rotation was +26°;
[0077] 3) Add 500g of the refined product of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com