Method for preparing benzoic acid esters
A technology of benzoic acid ester and benzoic acid, which is applied in the preparation of carboxylate, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of hindering acid discharge, impossible and inefficient reuse of benzoic acid, etc. Improved processability, reduced downtime, increased productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
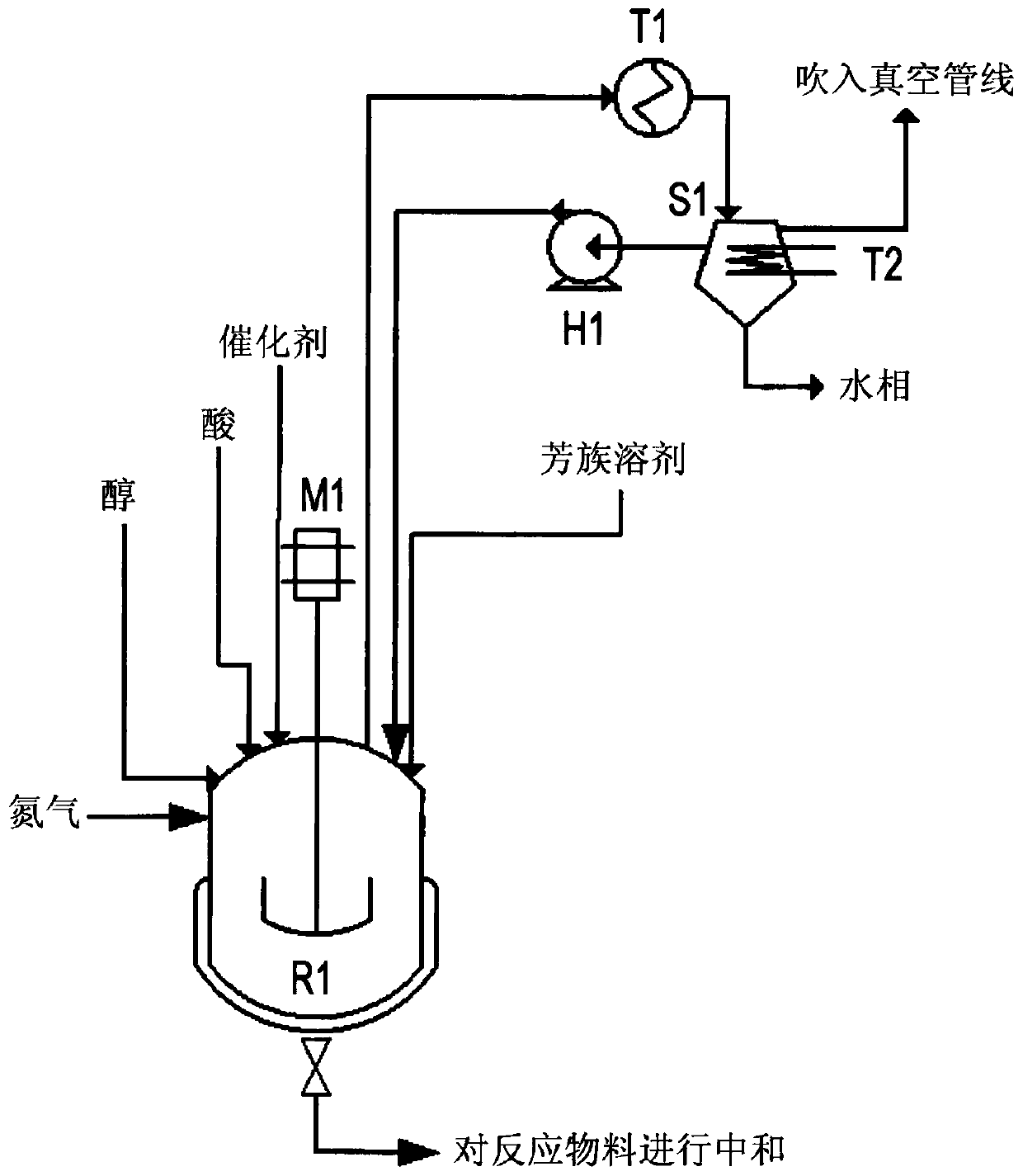
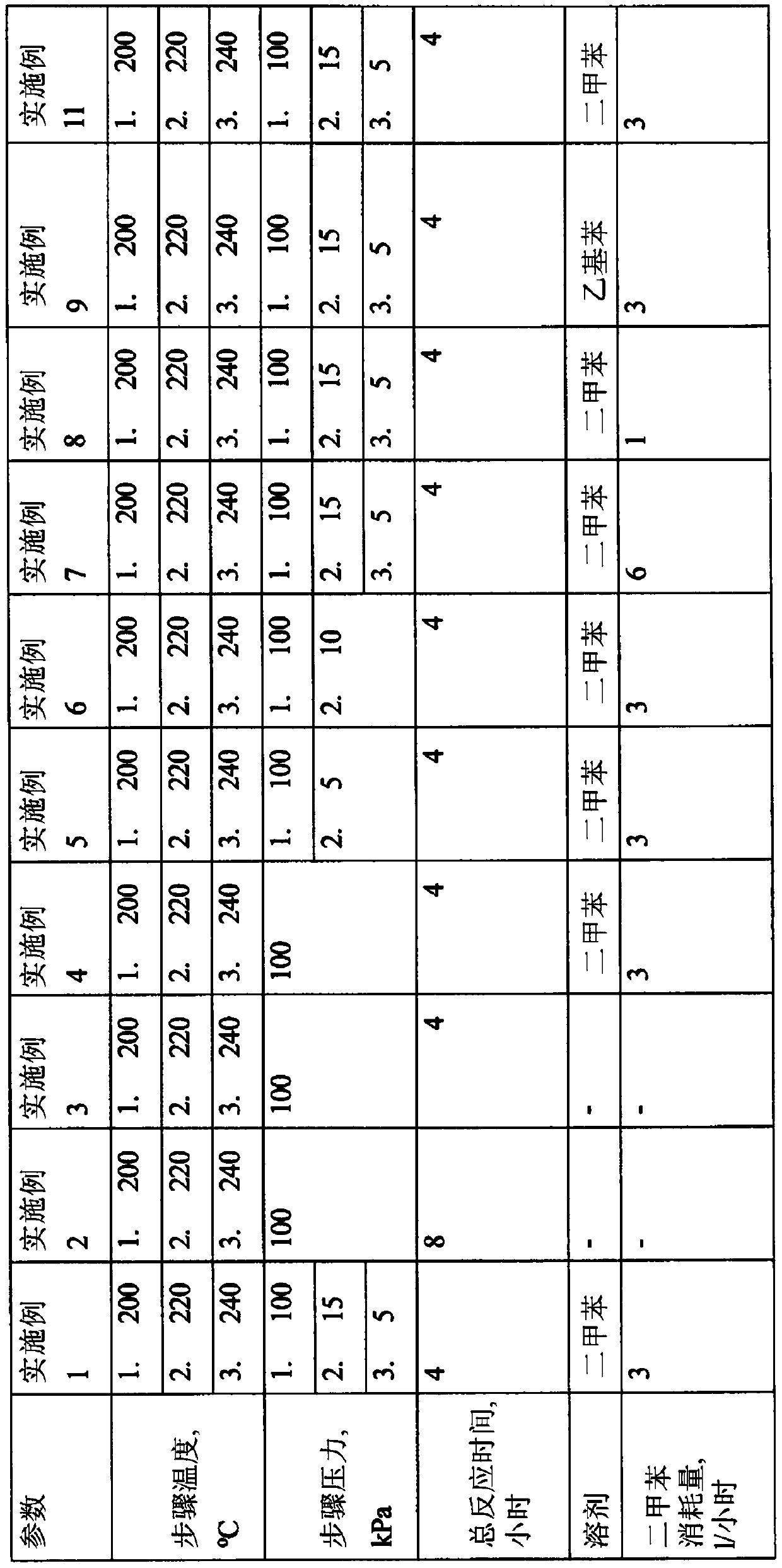
[0084] In a steel esterification reactor with a volume of 15 1 equipped with heating elements and stirring means, 3550 g of benzoic acid, 1500 g of diethylene glycol, and 13 g of titanium(IV) isopropoxide are added.
[0085] Xylene was added to the separator in an amount of 2500 g before the heat treatment. The reaction was carried out by constantly feeding xylene from the separator to the reaction mass at a rate of 3 1 / hour by means of a circulation pump. The reaction mass was heated to a temperature of 200°C, the temperature was increased to 220°C within one hour after the start of heating, and to 240°C within two hours after the start of heating.
[0086] Within 3 hours after starting the heating, the pressure in the reactor was reduced to 15 kPa, and within a further 30 minutes, the pressure in the reactor was reduced to 5 kPa. Within 4 hours after the start of heating, the reaction mass was cooled.
[0087] 4400 g of a reaction mass containing 0.5% by weight of benzoic ...
Embodiment 2
[0088] Embodiment 2 (comparative example)
[0089] In a steel esterification reactor with a volume of 15 1 equipped with heating elements and stirring means, 6500 g of benzoic acid, 2650 g of diethylene glycol and 23 g of titanium(IV) isopropoxide are added.
[0090] Xylene was added to the separator in an amount of 2500 g before the heat treatment. The reaction mass was heated to a temperature of 200°C. The esterification process was carried out under atmospheric pressure. The temperature was raised to 220°C within 4 hours of starting heating, to 240°C within 6 hours of starting heating, and the reaction mass was cooled within 8 hours after starting heating.
[0091] 8290 g of a reaction mass containing 7.8% by weight of benzoic acid, 9% by weight of diethylene glycol benzoate and 83.2% by weight of diethylene glycol dibenzoate were discharged from the esterification reactor. A phase comprising 856 g of water, 15 g of benzoic acid was discharged from the separator.
Embodiment 3
[0092] Embodiment 3 (comparative example)
[0093] This example was carried out according to Example 2, except that 4.5 g of titanium(IV) isopropoxide were added. The reaction mass was heated to a temperature of 200°C, the temperature was raised to 220°C within one hour after the start of heating, the temperature was raised to 240°C within two hours after the start of heating, and the temperature was raised to 240°C within 4 hours after the start of heating. The reaction mass is cooled.
[0094] 8255 g of a reaction mass comprising 7.6% by weight of benzoic acid, 4.5% by weight of diethylene glycol benzoate and 87.5% by weight of diethylene glycol dibenzoate were discharged from the esterification reactor. A phase comprising 878 g of water, 35 g of benzoic acid was discharged from the separator.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com