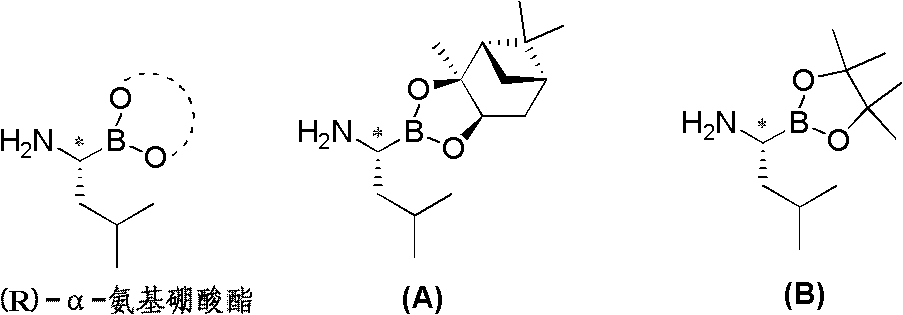
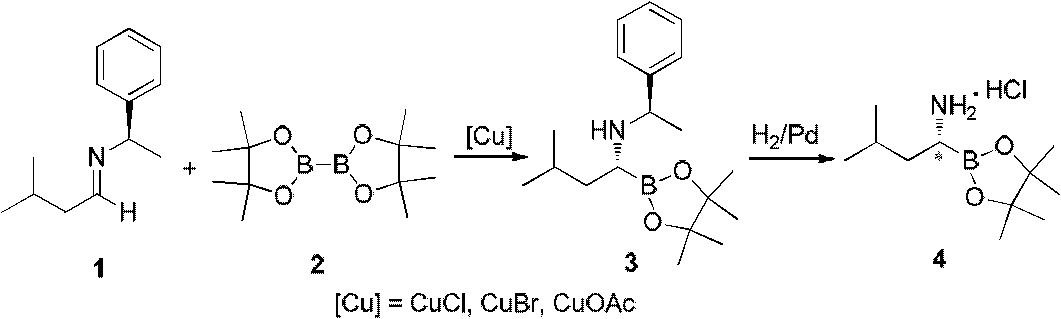
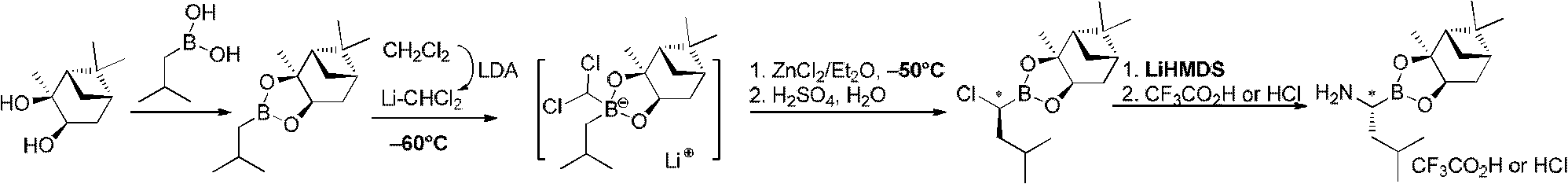Chiral alpha-amino boric acid esters, a preparation method and an application in the synthesis of bortezomib thereof
A technology of amino borate and amino, which is applied in the field of chiral α-amino borate intermediates, which can solve the problems of high energy consumption in reaction conditions and harsh production environment conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090]
[0091] 1.89 kg (10 mol) of (R)-2-methyl-N-(3-methylbutylene)propane-2-sulfinamide was added to a 20-liter reactor, and then 4 liters of tert-butyl methyl ether was added to stir and disperse evenly. Under the atmosphere of nitrogen protection, 2.72 kilograms (12mol) of neopentyl glycol diborate were added successively, and 329 grams of copper trifluorosulfonate (1mol) were added several times within 30 minutes. After the addition was completed, the reaction solution was allowed to react at room temperature. After 18 hours, TLC monitored the progress of the reaction. After the reaction was completed, 2 liters of ethyl acetate was added to disperse the reaction solution, and then 1N NaHCO was added. 3 3 liters of aqueous solution was washed once, the upper organic phase was washed three times with saturated brine, the aqueous phase was separated and discarded, and the organic phase was stirred and dried with 200 g of anhydrous sodium sulfate for at least 2 hours. The...
Embodiment 2
[0099]
[0100] Add 1.89 kg (10 mol) of (R)-2-methyl-N-(3-methylbutylene) propane-2-sulfinamide to a 20-liter reactor, then add 4 liters of tetrahydrofuran and stir to disperse and mix evenly. Under the atmosphere of protection, add 5.08 kilograms (20mol) of diboronic acid (2-methyl-2,4-pentanediol) respectively successively, add 177 grams of copper propionate (1.0mol) several times in 30 minutes, add , let the reaction solution react at 0°C for 48 hours, TLC monitors the progress of the reaction, after the reaction is completed, add 2 liters of ethyl acetate to disperse the reaction solution, and then add 1N NaHCO 3 3 liters of aqueous solution was washed once, and then the upper organic phase was washed three times with saturated brine, the aqueous phase was separated and discarded, and the organic phase was stirred and dried with 200 g of anhydrous sodium sulfate for at least 2 hours. The organic phase was concentrated under reduced pressure to obtain the compound R-N-(R...
Embodiment 3
[0108]
[0109] Add 1.89 kg (10 mol) of (R)-2-methyl-N-(3-methylbutylene) propane-2-sulfinamide to a 20-liter reactor, then add 4 liters of n-pentane and stir to disperse and mix evenly. Under the atmosphere of nitrogen protection, 3.38 kilograms (12mol) of diboronic acid (2,4-dimethyl-2,4-pentanediol) were added successively, and 221 grams of copper sulfate (1mol) were added several times within 30 minutes. , after adding, let the reaction solution react at room temperature for 18 hours, TLC monitors the progress of the reaction, after the reaction is completed, add 2 liters of ethyl acetate to disperse the reaction solution, then add 1N NaHCO 3 3 liters of aqueous solution was washed once, the upper organic phase was washed three times with saturated brine, the aqueous phase was separated and discarded, and the organic phase was stirred and dried with 200 g of anhydrous sodium sulfate for at least 2 hours. The organic phase was concentrated under reduced pressure to obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com