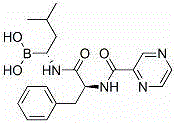
Bortezomib pharmaceutic composition for injection
A technology of bortezomib and composition, which is applied in the field of bortezomib pharmaceutical composition for injection, and can solve problems such as poor solubility and poor stability of bortezomib
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The following prescriptions were used: bortezomib 35 mg; cysteine 175 mg; mannitol 175 mg.
[0033] Preparation:
[0034] Add 2ml of tert-butanol to the raw material of bortezomib, ultrasonically dissolve it at room temperature for 5 minutes to completely dissolve, take the excipients and add 9ml of water to dissolve, combine with the above bortezomib solution, filter through a 0.22μm membrane and pack, each 1ml to 5ml Freeze-dry in neutral borosilicate glass freeze-drying vials, fill with nitrogen and press cap out of the box.
Embodiment 2
[0036] The following prescriptions were used: bortezomib 35mg; cysteine 175mg.
[0037] The preparation method is the same as in Example 1.
Embodiment 3
[0039] The following prescriptions were used: bortezomib 35mg; cysteine 350mg.
[0040] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com