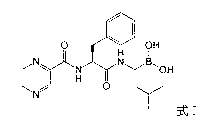
Freeze-dried composition containing bortezomib and preparation method of freeze-dried composition
A technology of bortezomib and composition, which is applied in the field of bortezomib-containing freeze-dried composition and its preparation, which can solve the problems of oxygen environment sensitivity and difficulty in dissolving the main drug, so as to improve the stability of the preparation and shorten the preparation time , the effect of avoiding contact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
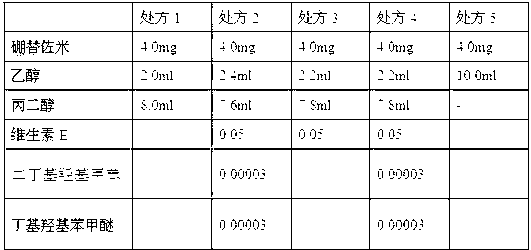
[0062] prescription:
[0063] Bortezomib 50mg Mannitol 1000mg tert-butanol 5g Water for Injection Dilute to 100ml
[0064] Preparation process: Heat tert-butanol to 26°C to make it melt into a liquid state, weigh 5g of tert-butanol, add water for injection to 50% of the total volume of the solution, mix well, fill with nitrogen for 0.5 hours; weigh 1000mg Add mannitol to the mixed solution, and stir until the mannitol is completely dissolved at 60°C to obtain an excipient solution; weigh 50 mg of bortezomib and add it to the excipient solution, stir until completely dissolved at 60°C, and add water for injection To the total volume of the preparation, the preparation intermediate solution is obtained, and the preparation process is filled with nitrogen for protection; after the preparation intermediate solution is qualified, it is filtered, filled according to the volume of 1ml, and sent to a freeze dryer for freeze-drying to obtain a freeze-...
Embodiment 2
[0068] prescription:
[0069] Bortezomib 500mg Mannitol 2500mg tert-butanol 50g Water for Injection Dilute to 100ml
[0070] Preparation process: Heat tert-butanol to 50°C to make it melt into a liquid state, weigh 50g of tert-butanol, add water for injection to 95% of the total volume of the solution, mix well, fill with nitrogen for 2 hours; weigh 2500mg Add mannitol to the mixed solution, and stir until the mannitol is completely dissolved at 50°C to obtain an excipient solution; weigh 500 mg of bortezomib and add it to the excipient solution, stir until completely dissolved at 50°C, and add water for injection To the total volume of the preparation, the preparation intermediate solution is obtained, and the preparation process is filled with nitrogen for protection; after the preparation intermediate solution is qualified, it is filtered, filled according to the volume of 1ml, and sent to a freeze dryer for freeze-drying to obtain a freez...
Embodiment 3
[0075] prescription:
[0076] Bortezomib 175mg Mannitol 1750mg tert-butanol 40g Water for Injection Dilute to 100ml
[0077] Preparation process: Heat tert-butanol to 40°C to make it melt into a liquid state, weigh 40g of tert-butanol, add water for injection to 90% of the total volume of the solution, mix well, and fill with nitrogen for 1 hour; weigh 1750mg Add mannitol to the mixed solution, and stir until the mannitol is completely dissolved at 40°C to obtain an excipient solution; weigh 175 mg of bortezomib and add it to the excipient solution, stir until completely dissolved at 40°C, and add water for injection To the total volume of the preparation, the preparation intermediate solution is obtained, and the preparation process is filled with nitrogen for protection; after the preparation intermediate solution is qualified, it is filtered, filled according to the volume of 2ml, and sent to a freeze dryer for freeze-drying to obtain a fr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com