Methods and compositions useful for treating diseases involving bcl-2 family proteins with quinoline derivatives
a technology of bcl-2 family proteins and quinoline derivatives, which is applied in the field of methods and compositions for treating cancer and autoimmune diseases, can solve the problems of blocking the sensitivity of tumor cells to cytostatic or apoptosis inducing drugs, poor survival prognosis of patients with cll, and high cos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
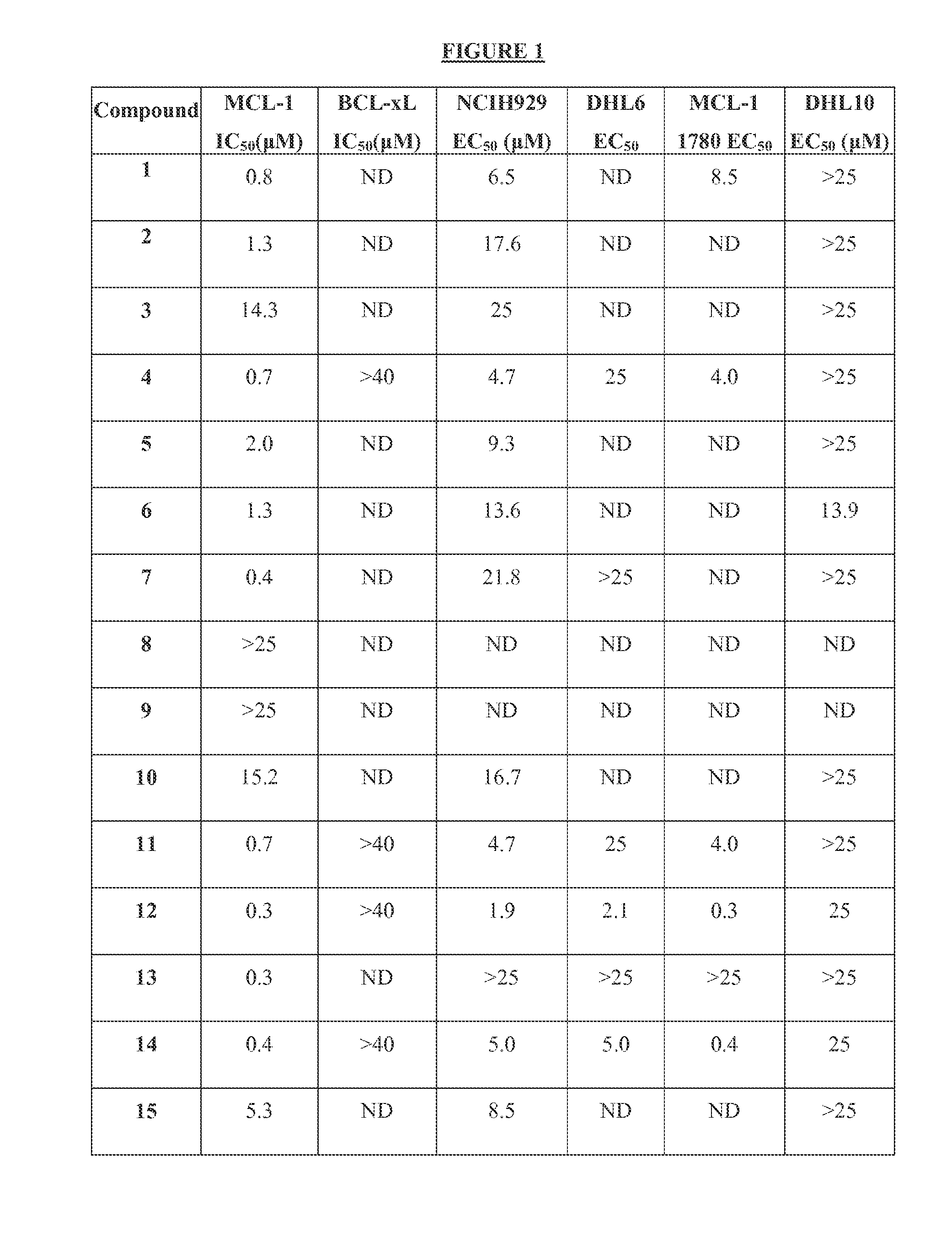
Inhibition of Mcl-1 by Compounds of Formula I-III
[0297]In this example, a Fluorescence Polarization (FP) assay was used to demonstrate the activity of the Mcl-1 inhibitors described in Formulas I-III in inhibiting Mcl-1 as well as Bcl-xL binding to Bim BH3 as described in Degterev et al. (2001) Nature Cell Biology 3: 173-182.
[0298]A nineteen amino acid peptide, corresponding to the BH3 domain of Bim, with the sequence FITC-GGGIAQELRRIGDEFNAY (SEQ ID NO: 14) was labeled with the fluorophore FITC according to the manufacturer's instructions (Molecular Probes, Eugene, Oreg.). This sequence was identified as being able to bind to purified Bcl-xL protein (Sattler et al. (1997) Science 275(5302): 983-86, which is hereby incorporated by reference in its entirety) and to have biological activity in cells (Holinger et al. J. Biol. Chem. 274: 13298-1330, which is hereby incorporated by reference in its entirety).
[0299]In addition, recombinant GST-Mcl-1 and GST-Bcl-xL fusion proteins were gene...
example 2
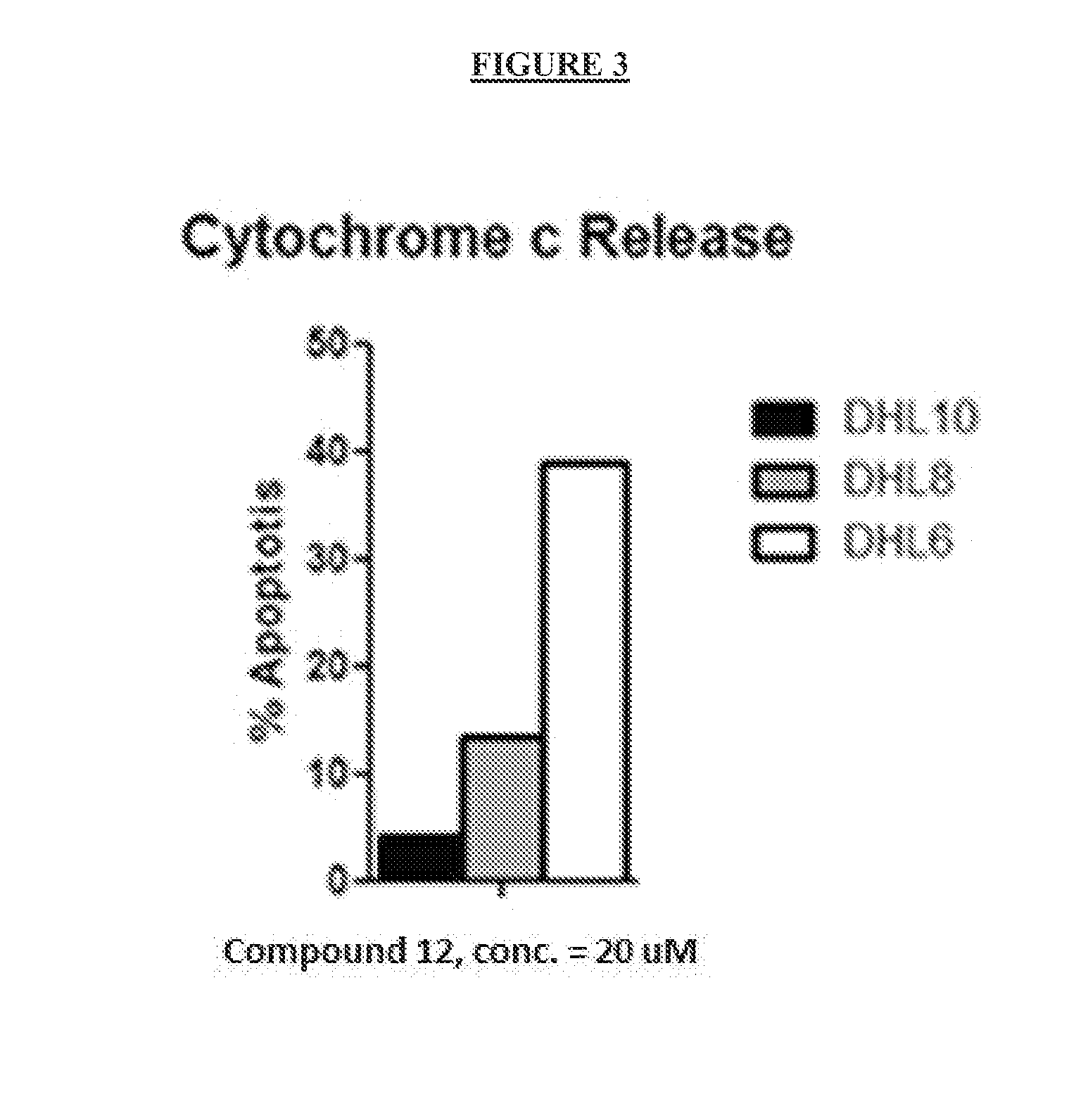
Activity of Compounds of Formulae I, II, or III in Killing Mouse and Human Tumor Cell Lines
[0302]This example demonstrates the activity of the compounds of Formula I-III and derivatives, in killing certain human tumor-derived cell lines grown in culture. Leukemia and myeloid cells used to assess cell tumor killing activity of the compounds are described. Compounds active in these cell lines have good potential as therapies to treat leukemia and myeloid cancers.
Materials and Method
[0303]Cell Culture
[0304]The lymphoid derived cell lines DHL-6, DHL-8, and DHL-10 were obtained from Anthony Letai of the Dana Farber Cancer Research Institute, Boston, Mass. The myeloid derived cell line NCI-H929 was obtained from the NIH / NCI cell repository. The mouse leukemia-derived cell line MCL-1-1780 (Ryan et al., Proc. Nat. Acad. Sci USA, 107, 12895-12900, which is hereby incorporated by reference in its entirety) was obtained from Anthony Letai of the Dana Farber Cancer Research Institute, Boston, M...
example 3
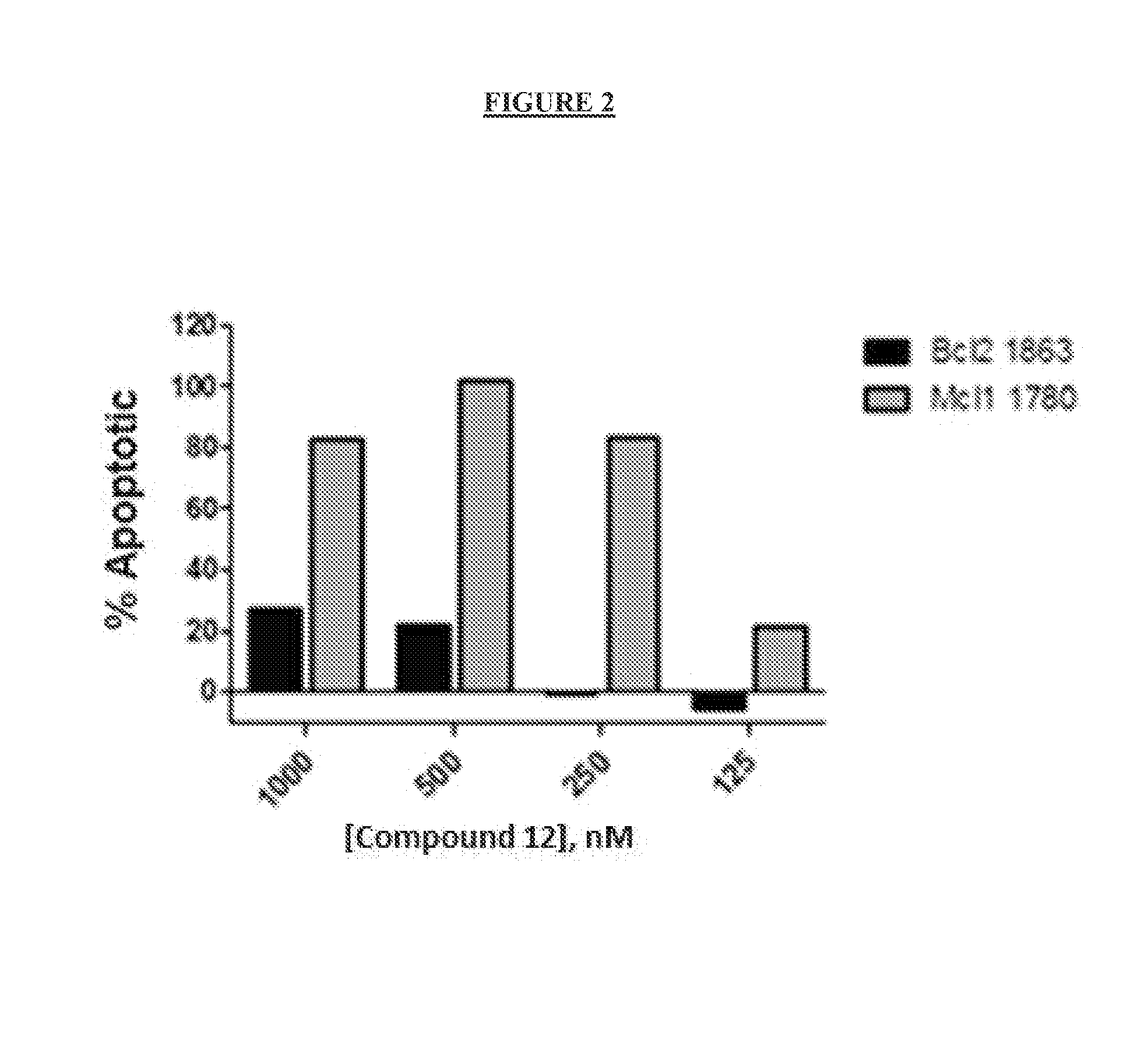
Fluorescence-based cell-based cytotoxicity assay using 4′-6-diamidino-2-phenylindole (DAPI)
[0309]Materials and Methods
[0310]The mouse leukemia-derived cell lines Mcl-1-1780 and Bcl-2-1863 (Ryan et al., Proc. Nat. Acad. Sci USA, 107, 12895-12900, which is hereby incorporated by reference in its entirety) were obtained under license from Dana Farber Cancer Institute. Cells were grown in RPMI 1640 medium (GIBCO-BRL) with 2 mM L-glutamine, 4.5 g / L glucose, 1.0 mM sodium pyruvate and 5% fetal bovine serum. Cells were expanded in tissue culture in appropriate media and then sub-cultured into 96-well plates at a seeding density of 20,000 cells per well. After incubation for 24 hours, cells were treated with compounds that are titrated into appropriate medium with FCS. Four concentrations of compound were used: 1 μM, 500 nM, 250 nM, and 125 nM. Assay was run in triplicate for each compound at each concentration and averages were calculated. Cells were treated with compound for 24 hours, the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com