Method for synthesizing bortezomib
A synthesis method and bortezomib technology are applied in the field of bortezomib synthesis, and can solve the problems of affecting the purity of the bortezomib finished product, being difficult to bortezomib, and the high cost of a condensing agent, achieving low price, few reaction steps, and high efficiency. The effect of purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
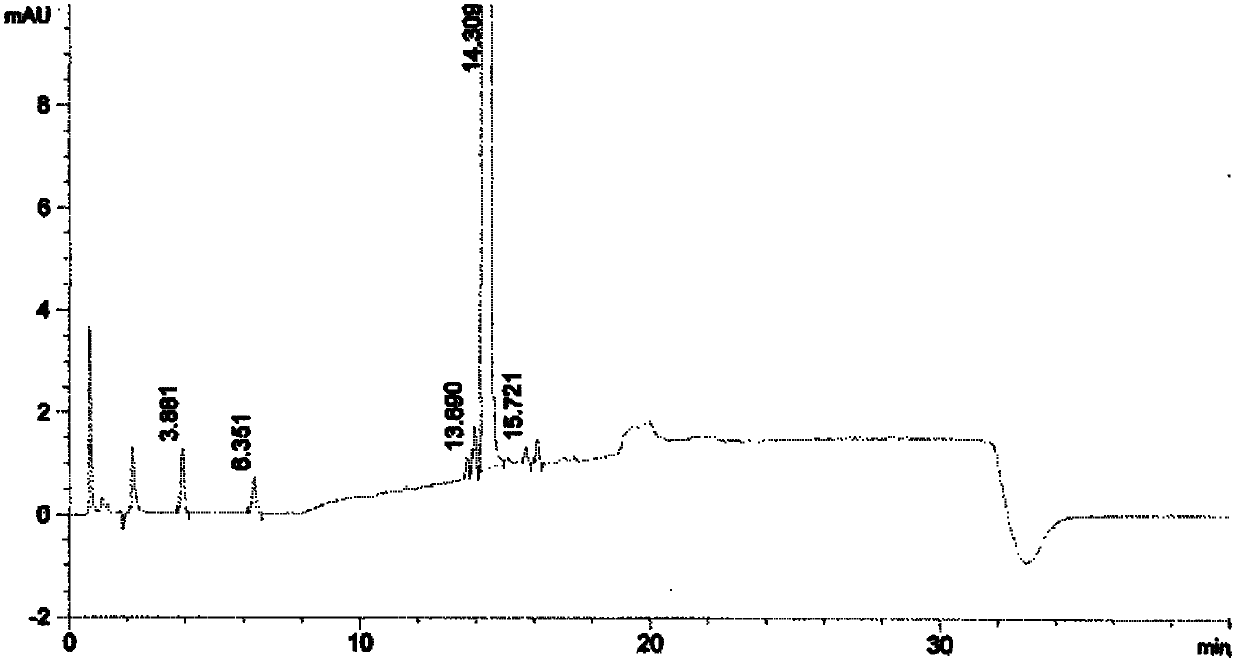
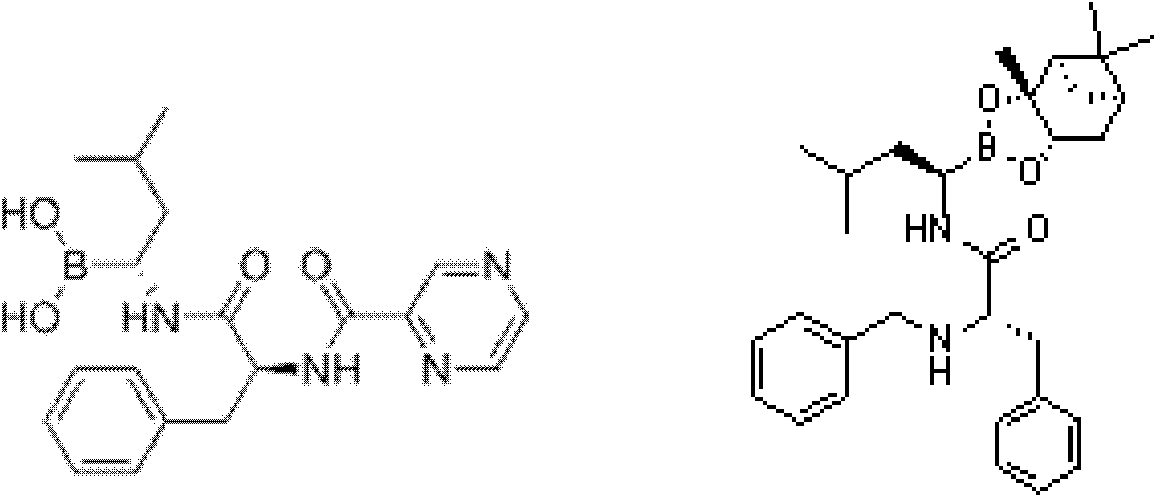
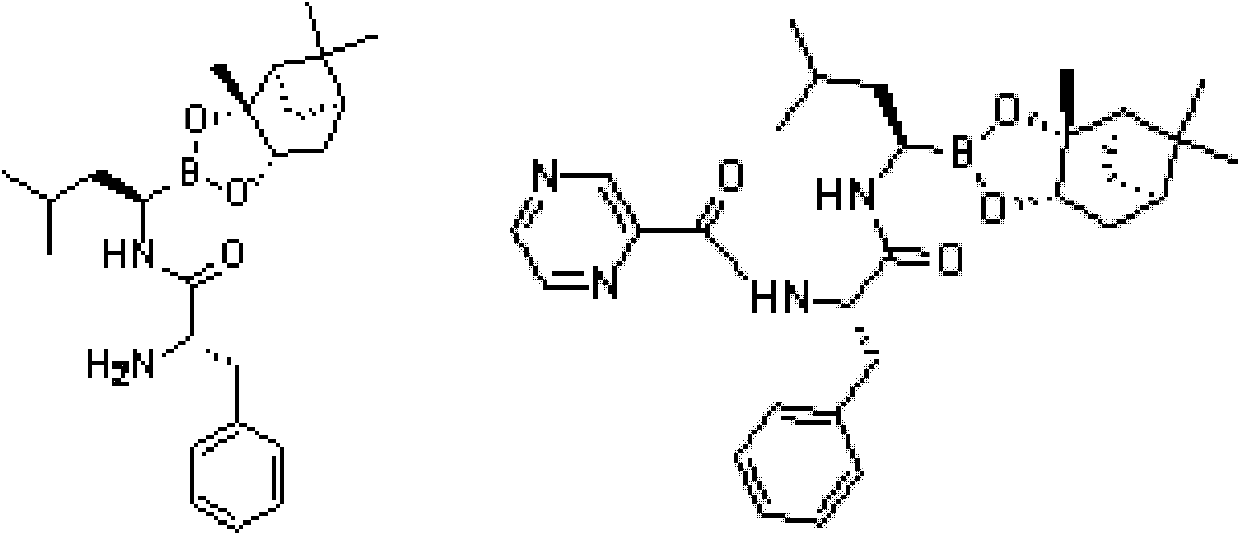
[0055] Under the protection of nitrogen, 24.5g (0.1mol) 2(s) benzylamino-3-phenylpropionic acid and 6.22g N-methylmorpholine were added to 130mL freshly prepared THF, cooled to -20°C and poured into it Add 8.4g of isobutyryl chloride, after stirring for 0.5 hours, add dropwise 38g (0.1mol) (aR, 3aS, 4S, 6S, 7aR)-hexahydro-3a,8,8-trimethyl-alpha at 20°C -(2-methylpropyl)-4,6-methylbridge-1,3,2-benzodioxaborane-2-methylamine 2,2,2-trifluoroacetate solution, followed by Add 16mL of N,N-diisopropylethylamine to adjust the pH to 9-10, then raise the temperature to room temperature and stir for 6 hours under the protection of nitrogen. After the reaction is completed, concentrate to dryness under reduced pressure, and add 340mL of methyl tert-butyl to the residue base ether, and washed twice with 175 mL of 1.5% hydrochloric acid. The acid layer was back-extracted with methyl tert-butyl ether, the organic phase was washed once with 175 mL of water and saturated sodium chloride succe...
Embodiment 2
[0061] Under the protection of nitrogen, 24.5g (0.1mol) 2(s) benzylamino-3-phenylpropionic acid and 6.22g N-methylmorpholine were added to 130mL freshly prepared THF, cooled to -20°C and poured into it Add 8.4g of isobutyryl chloride, after stirring for 0.5 hours, add dropwise 38g (0.1mol) (aR, 3aS, 4S, 6S, 7aR)-hexahydro-3a,8,8-trimethyl-alpha at 20°C -(2-methylpropyl)-4,6-methylbridge-1,3,2-benzodioxaborane-2-methylamine 2,2,2-trifluoroacetate solution, followed by Add 16mL of N,N-diisopropylethylamine to adjust the pH to 10-11, then raise the temperature to room temperature and stir for 4 hours under nitrogen protection. After the reaction is completed, concentrate to dryness under reduced pressure, and add 340mL of methyl tert-butyl to the residue base ether, and washed twice with 175 mL of 1.5% hydrochloric acid. The acid layer was back-extracted with methyl tert-butyl ether, the organic phase was successively washed once with 175 mL of water and saturated sodium chlorid...
Embodiment 3
[0067] Under the protection of nitrogen, 24.5g (0.1mol) 2(s) benzylamino-3-phenylpropionic acid and 6.22g N-methylmorpholine were added to 130mL freshly prepared THF, cooled to -20°C and poured into it Add 8.4g of isobutyryl chloride, after stirring for 0.5 hours, add dropwise 38g (0.1mol) (aR, 3aS, 4S, 6S, 7aR)-hexahydro-3a,8,8-trimethyl-alpha at 20°C -(2-methylpropyl)-4,6-methylbridge-1,3,2-benzodioxaborane-2-methylamine 2,2,2-trifluoroacetate solution, followed by Add 16mL of N,N-diisopropylethylamine to adjust the pH to 11~12, then raise the temperature to room temperature and stir for 3 hours under nitrogen protection. After the reaction is completed, concentrate to dryness under reduced pressure, and add 340mL of methyl tert-butyl to the residue base ether, and washed twice with 175 mL of 1.5% hydrochloric acid. The acid layer was back-extracted with methyl tert-butyl ether, the organic phase was washed once with 175mL water and saturated sodium chloride successively, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com