Method for synthesizing valeraldehyde through butene hydroformylation
A technology for synthesizing valeraldehyde and butene by hydroformylation of butene is applied in chemical instruments and methods, carbon monoxide reaction preparation, organic compound/hydride/coordination complex catalyst, etc. problems such as low activity and selectivity, to achieve the effect of simple separation, less investment in operating equipment, and strong industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
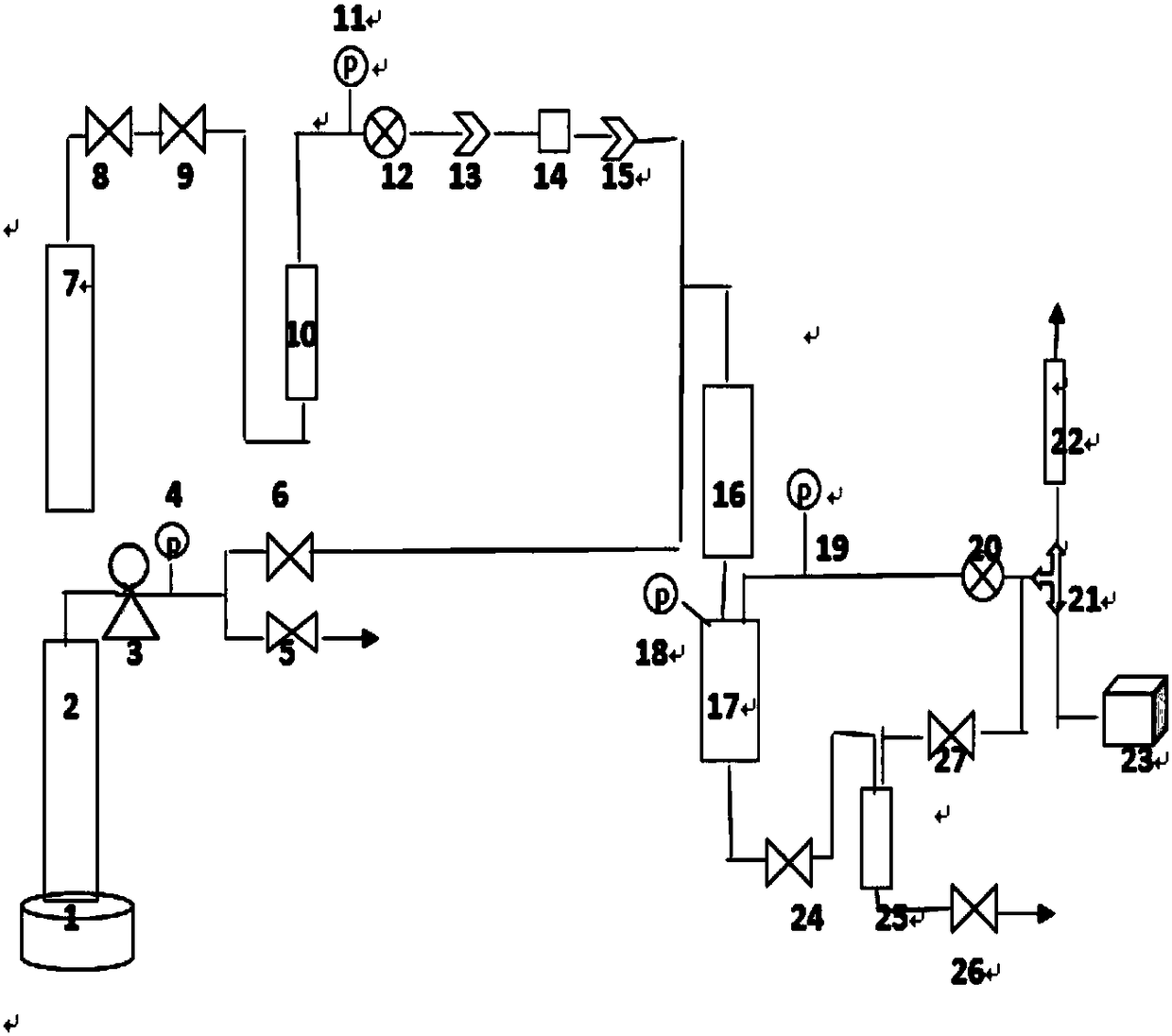
Image
Examples
Embodiment 1
[0027] Under 298K and inert gas protection atmosphere, 0.05gRh(acac)(CO) 2 Dissolve in 100ml tetrahydrofuran solvent, add 1g tris(4-vinylphenyl) phosphine, stir the polymer at 298K and an inert gas atmosphere for 24h, then vacuum the solvent at 333K to obtain Organic polymer-supported heterogeneous catalyst for hydroformylation, denoted as 2%Rh / POL-PPh 3 .
Embodiment 2
[0029] Under 298K and inert gas protection atmosphere, 0.0035gRh(acac)(CO) 2 Dissolve in 75ml tetrahydrofuran solvent, add 1g tris(4-vinylphenyl) phosphine, stir the polymer at 298K and an inert gas atmosphere for 24h, then vacuum the solvent at 333K to obtain Organic polymer-supported heterogeneous catalyst for hydroformylation, denoted as 0.125% Rh / POL-PPh 3 .
Embodiment 3
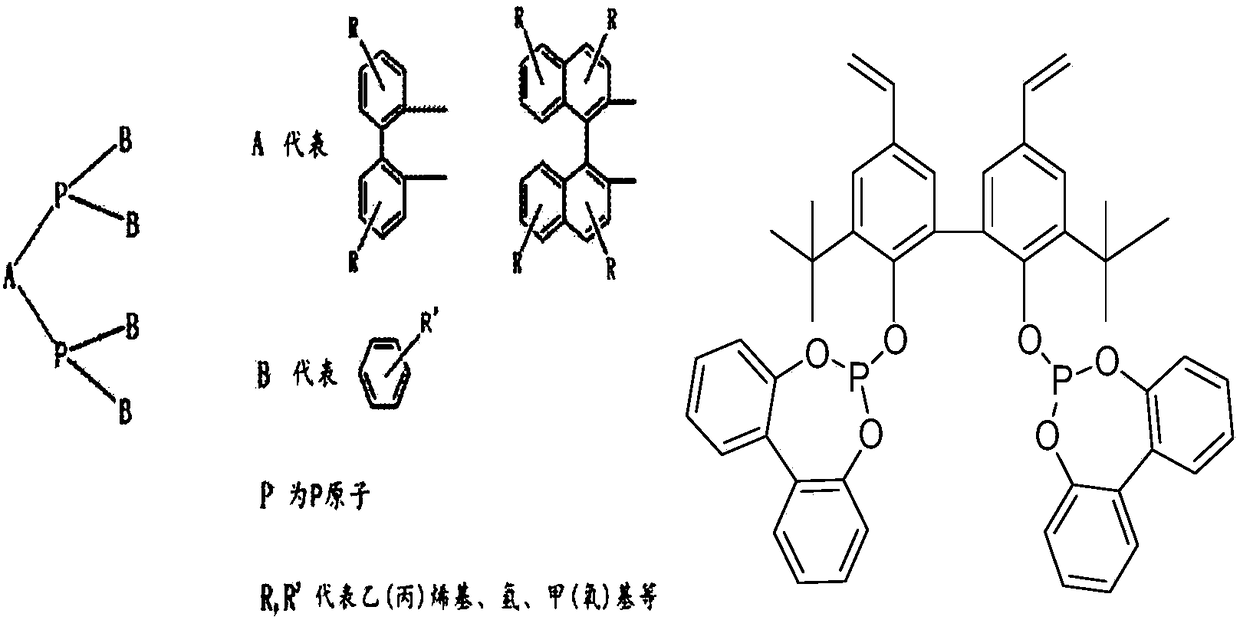
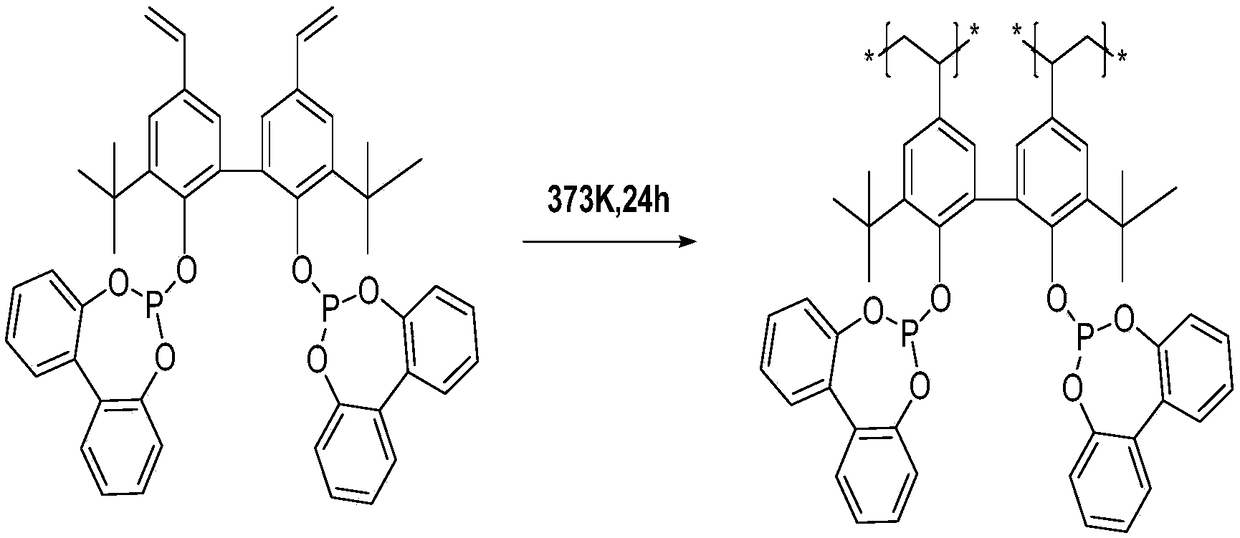
[0031] Under 298K and an inert gas protection atmosphere, 10.0 grams of Vinyl Biphephos monomer (attached figure 1 ) was dissolved in 100.0ml tetrahydrofuran solvent, and 2.5g comonomer tris (4-vinylphenyl) phosphine (L1) was added simultaneously; Hour. The stirred solution was moved to an autoclave, and polymerized by solvothermal polymerization at 373K and an inert gas atmosphere for 24 hours. After the above polymerized solution is cooled to room temperature, the solvent is removed under vacuum at room temperature to obtain an organophosphine mixed polymer carrier copolymerized with Vinyl Biphephos and tris(4-vinylphenyl)phosphine organic monomer. figure 2 It is a schematic diagram of Vinyl Biphephos organic mixed polymer carrier polymerization technology route. Take by weighing 3.13 milligrams of tricarbonyl rhodium acetylacetonate and be dissolved in 10.0 ml of tetrahydrofuran solvent, add 1.0 gram of organic mixed polymer carriers obtained by copolymerization of Vinyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com