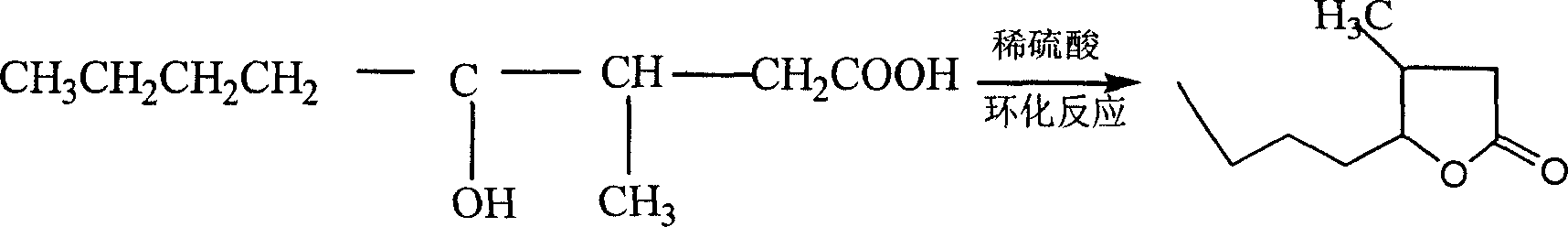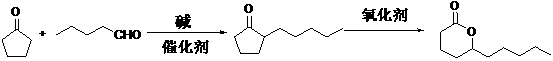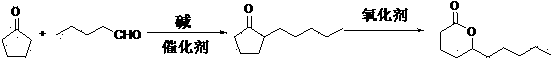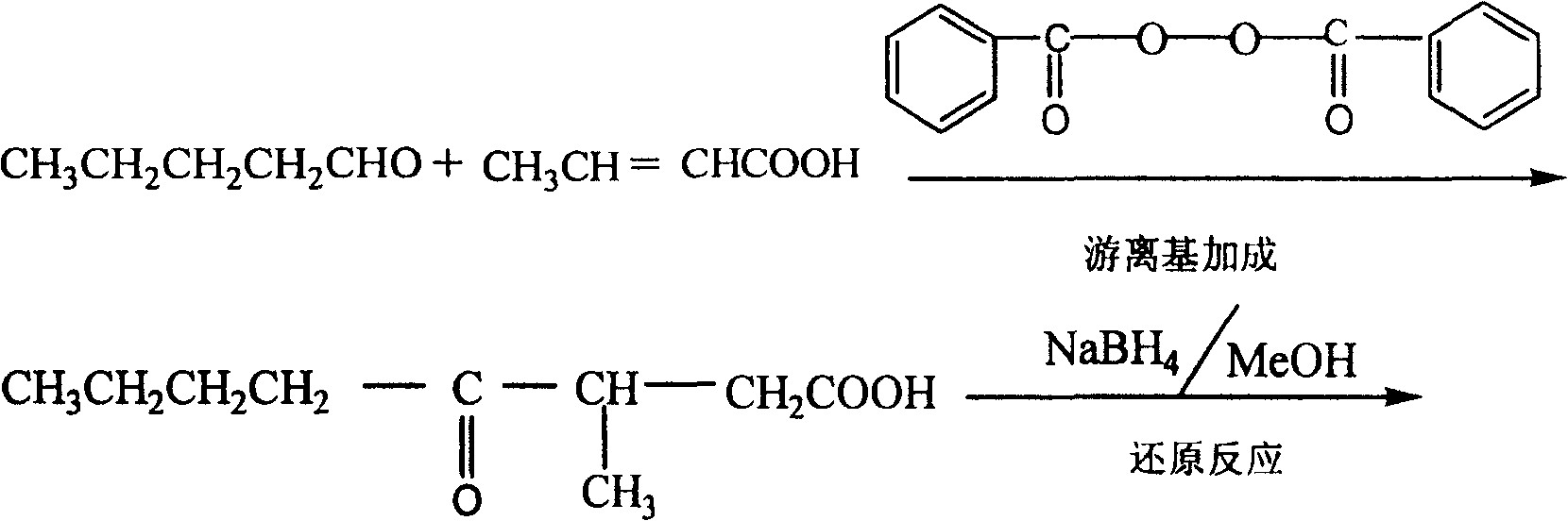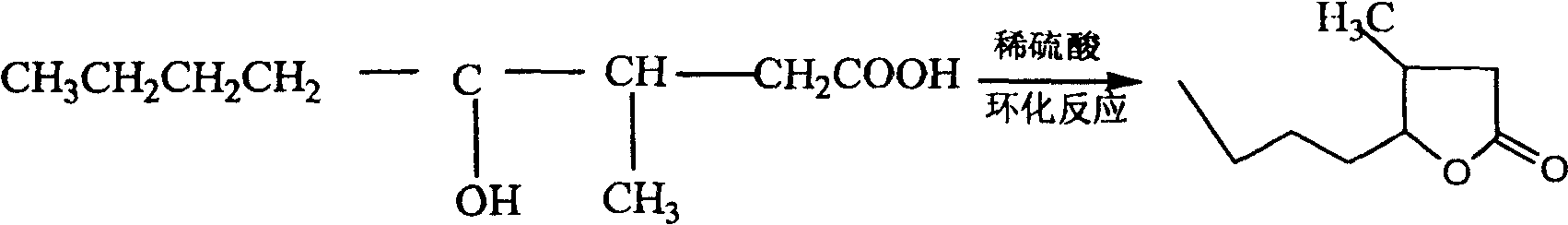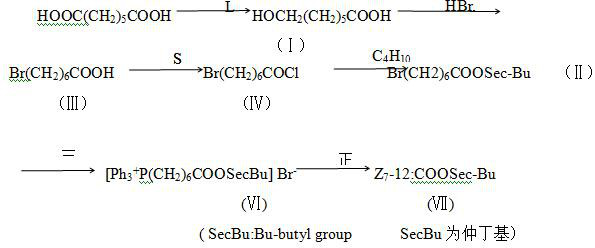Patents
Literature
37 results about "N-valeraldehyde" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Testing has not been completed to determine the carcinogenicity of ... n-valeraldehyde, ... /a/ related low-molecular-weight-aldehyde. However, the limited studies to date indicate that ... /this substance has/ chemical reactivity and mutagenicity similar to acetaldehyde and malonaldehyde .
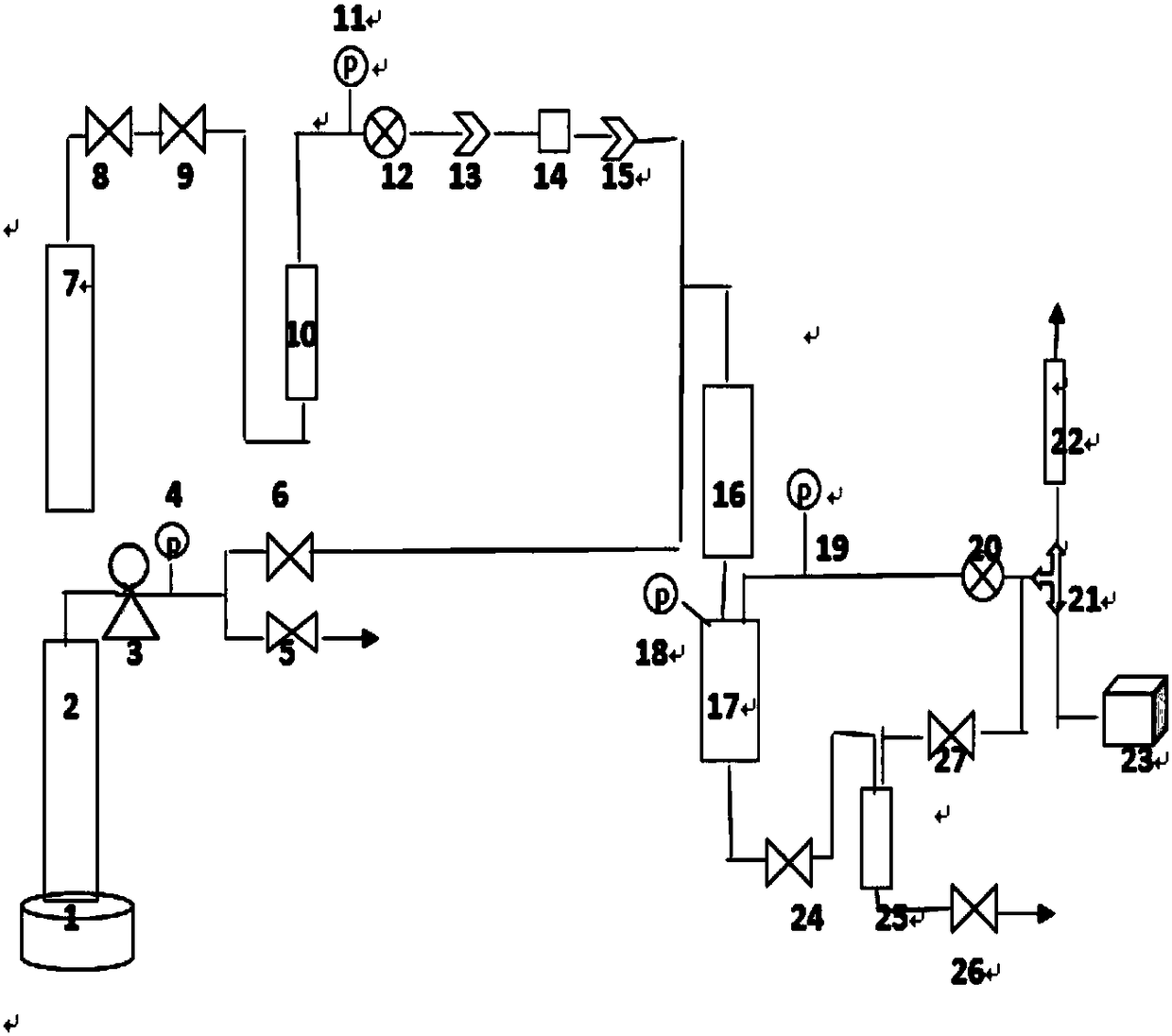
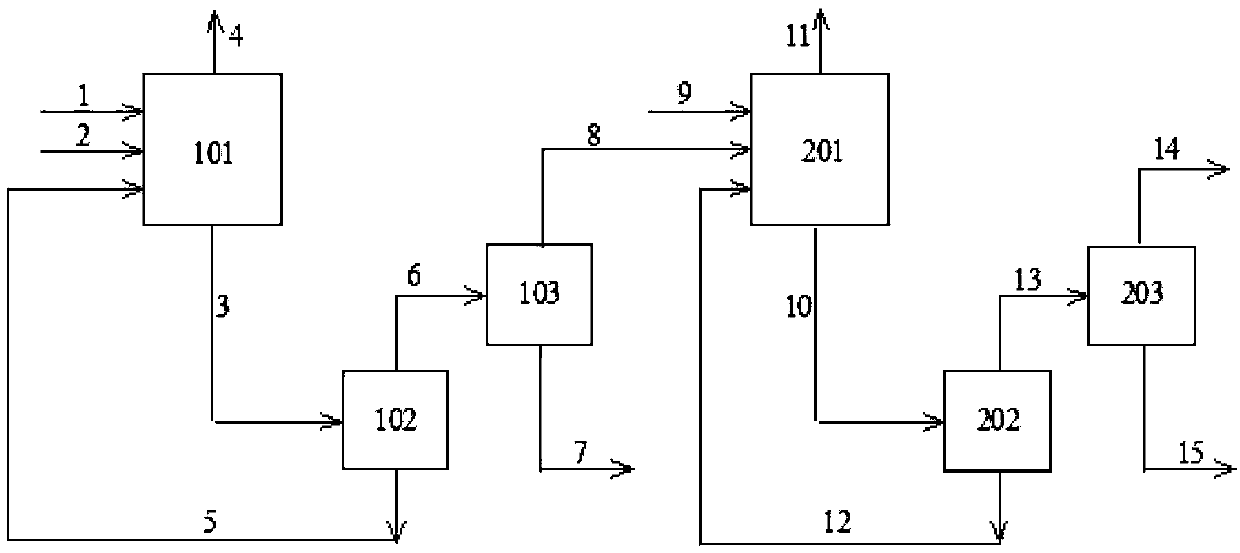
Method for preparing aldehyde through linear chain olefin hydroformylation
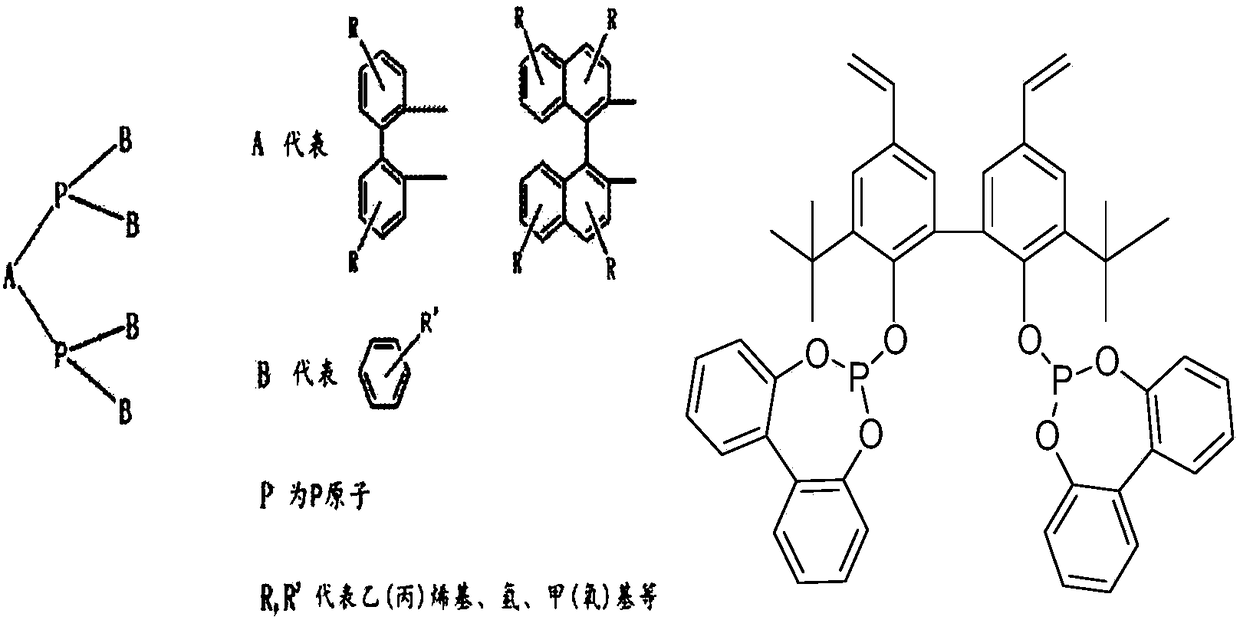
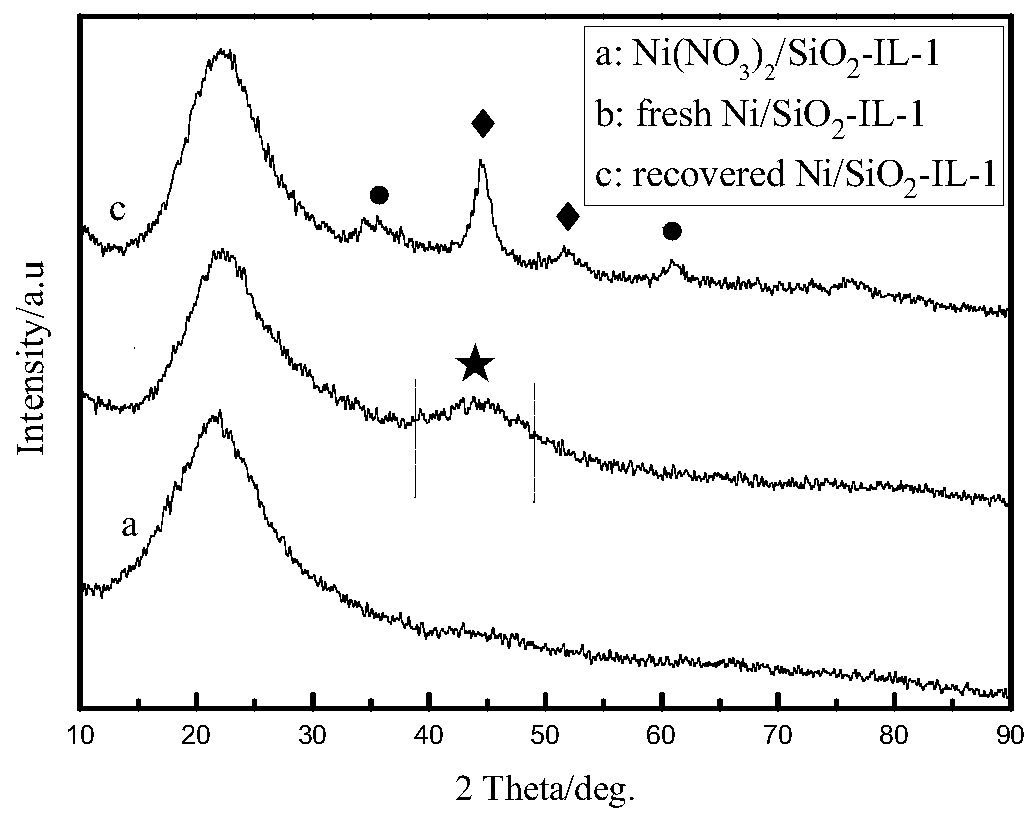
InactiveCN102911021AReduce dosageGuaranteed uptimeOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by carbon monoxide reactionFormylation reactionDiphosphines
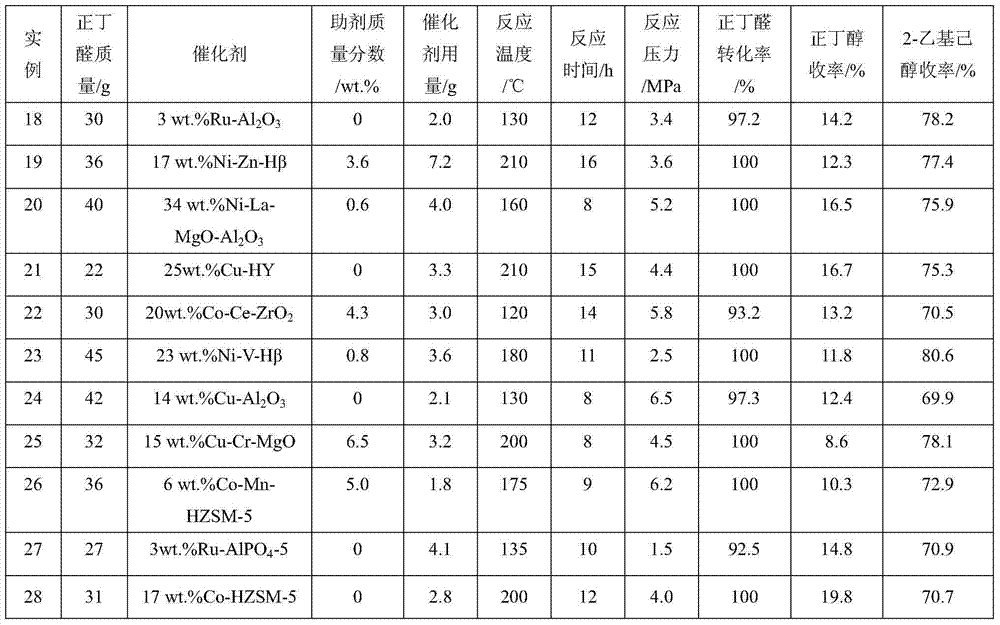
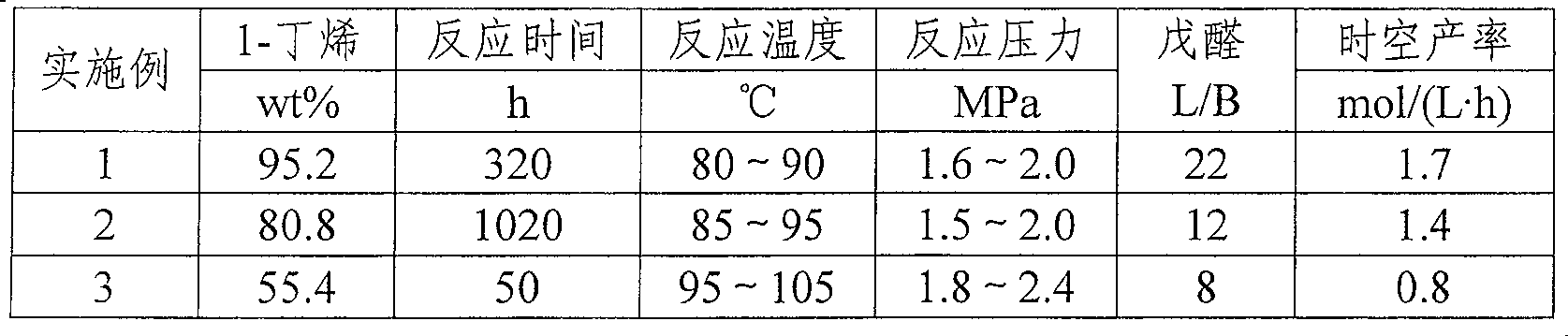
The invention relates to a method for preparing aldehyde through linear chain olefin hydroformylation. According to the method, a continuous reaction mode is used for performing olefin hydroformylation reaction in a homogeneous catalyst system; a catalyst is a composite catalysis system composed of a rhodium complex, a biphenyl backbone or biphenyl backbone diphosphine ligands and triphenylphosphine or triphenyl phosphate monophosphorous ligands; a reaction solvent comprises butyraldehyde, valeraldehyde, toluene or isodecanol; when the catalyst system uses propylene or butene-1 as raw materials under the condition of a low molar ratio of the diphosphine ligands to the rhodium complex, contents of the aldehyde generated by hydroformylation of the propylene and the butene-1 is larger than 97% and 95% respectively; and when the catalyst system uses a mixture of the butene-1 and butene-2 as raw materials, and the content of n-valeraldehyde in hydroformylation reaction products can reach above 85%.
Owner:QINGDAO SANLI BENNUO CHEM IND +1
Method for producing epoxy cyclohexane
The epoxy cyclohexane producing process includes using cyclohexene as main material, molecular oxygen as oxygen source, n-valeraldehyde or isovaleraldehyde or isobutylaldehyde as intermediate; adopting re-compounded catalyst including at least one oxide of Mn, Fe, Co and Ni, at least one oxide of Mo and W, and at least one oxo acid of N, P and As; and reaction at 30-80 deg.c for 2-12 hr. The re-compounded catalyst can oxidize aldehyde into per-acid in high selectivity and catalyze the reaction between per-acid with cyclohexene in high selectivity to obtain epoxy cyclohexane as the destination product in the same reactor, with the single-path cyclohexene converting rate reaching 23 % and selectivity reaching 98 %. The reaction process uses no solvent and the metal oxide in the re-compounded catalyst may be reused.
Owner:BALING PETRO CHEM CO LTD SINOPEC
Synthetic method of diamondback moth sex pheromone compound
InactiveCN102795997ALow priceReduce manufacturing costOxygen-containing compound preparationOrganic compound preparationHydrolysisToxicology
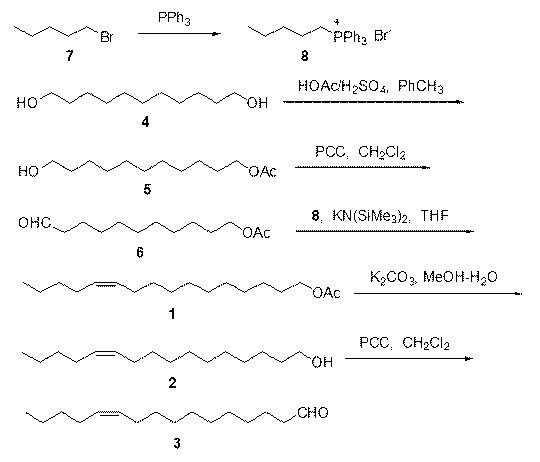
The invention discloses two synthetic methods of a diamondback moth sex pheromone compound. The first method adopts 1,11-undecadiol and n-amyl bromide as initial raw materials, and comprises four-step reactions including a critical Wittig reaction to synthesize (Z)-11-hexadecen-1-yl acetate, a hydrolytic reaction to obtain (Z)-11-hexadecen-1-ol, and a pyridinium chlorochromate oxidation reaction to obtain (Z)-11-hexadecenal. The second method adopts 1,11-undecadiol and valeraldehyde as initial raw materials, and comprises three-step reactions including a critical Wittig reaction to synthesize (Z)-11-hexadecen-1-ol, a pyridinium chlorochromate oxidation reaction to obtain (Z)-11-hexadecenal, and an esterification reaction to obtain (Z)-11-hexadecen-1-yl acetate. The invention has the advantages of cheap and easily available raw materials, simple synthetic route, mild reaction condition, convenient and safe operation, high yield, and low cost.
Owner:昆明博鸿科技有限公司
Method for synthesizing valeraldehyde through butene hydroformylation
ActiveCN108069842AEasy to separateLess investmentOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by carbon monoxide reactionButeneFormylation reaction
The invention relates to a method for synthesizing valeraldehyde through butene hydroformylation, and particularly provides a phosphorus-containing organic polymer supported heterogeneous catalysis method for synthesizing valeraldehyde through butene hydroformylation. The method is characterized by comprising steps as follows: liquid butene is metered by an electronic metering balance and continuously enters a reactor with synthesis gas, a hydroformylation reaction is performed under the action of a phosphorus-containing organic polymer supported heterogeneous catalyst, and a valeraldehyde product continuously flows out of the reactor and then is separated from the catalyst and produced continuously. The method has the characteristics of high catalytic activity and good product selectivity, almost no butane is contained in the product, and a molar ratio of n-valeraldehyde to isovaleraldehyde can reach 2-65; besides, the catalyst is good in stability, the product and the catalyst can achieve the excellent-performance characteristics of being simple to separate and the like, then industrial low-cost production of a butene hydroformylation product is realized, and new idea and technical guidance are provided for industrialization of the butene hydroformylation.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
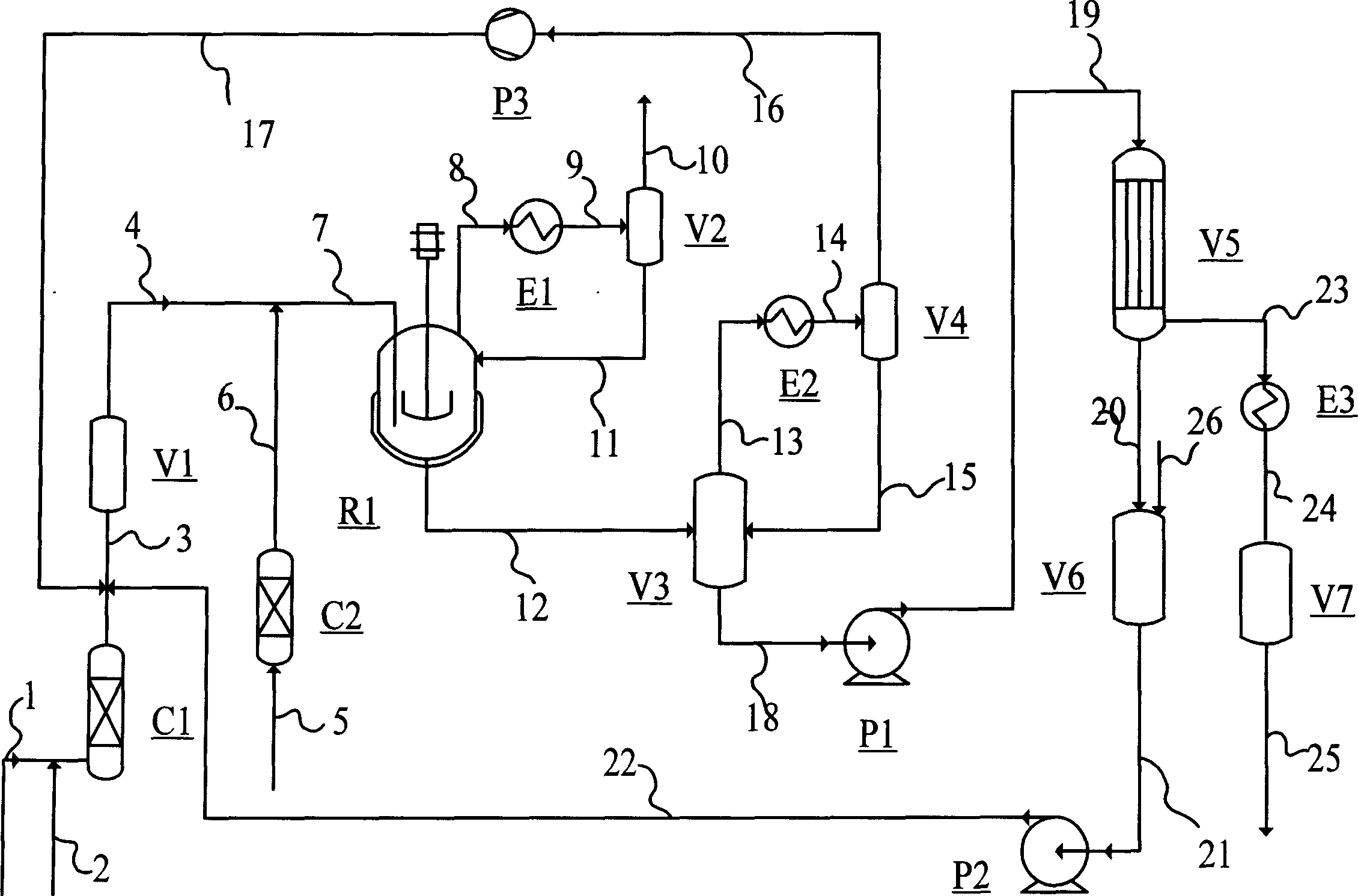
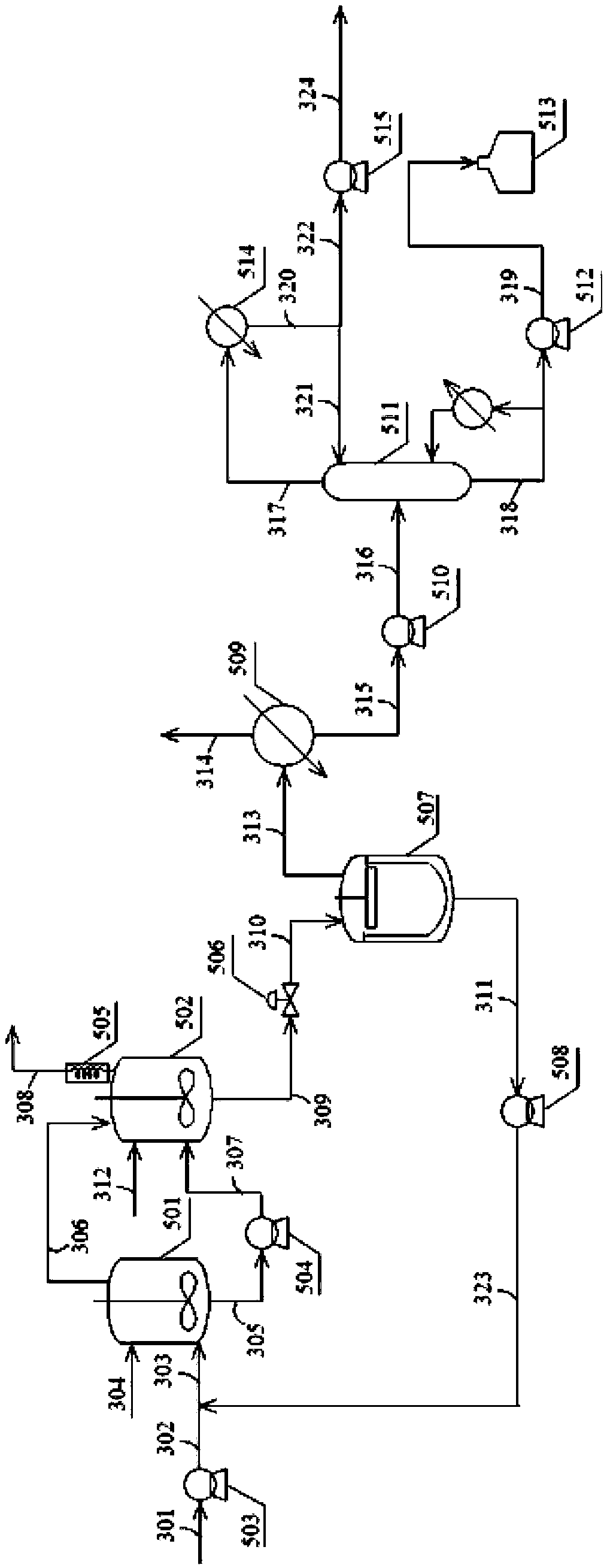
Process of continuously preparing n-pentanal
ActiveCN101054342AHigh activityHigh regional selectivityPreparation by carbon monoxide reactionBoiling pointEvaporator
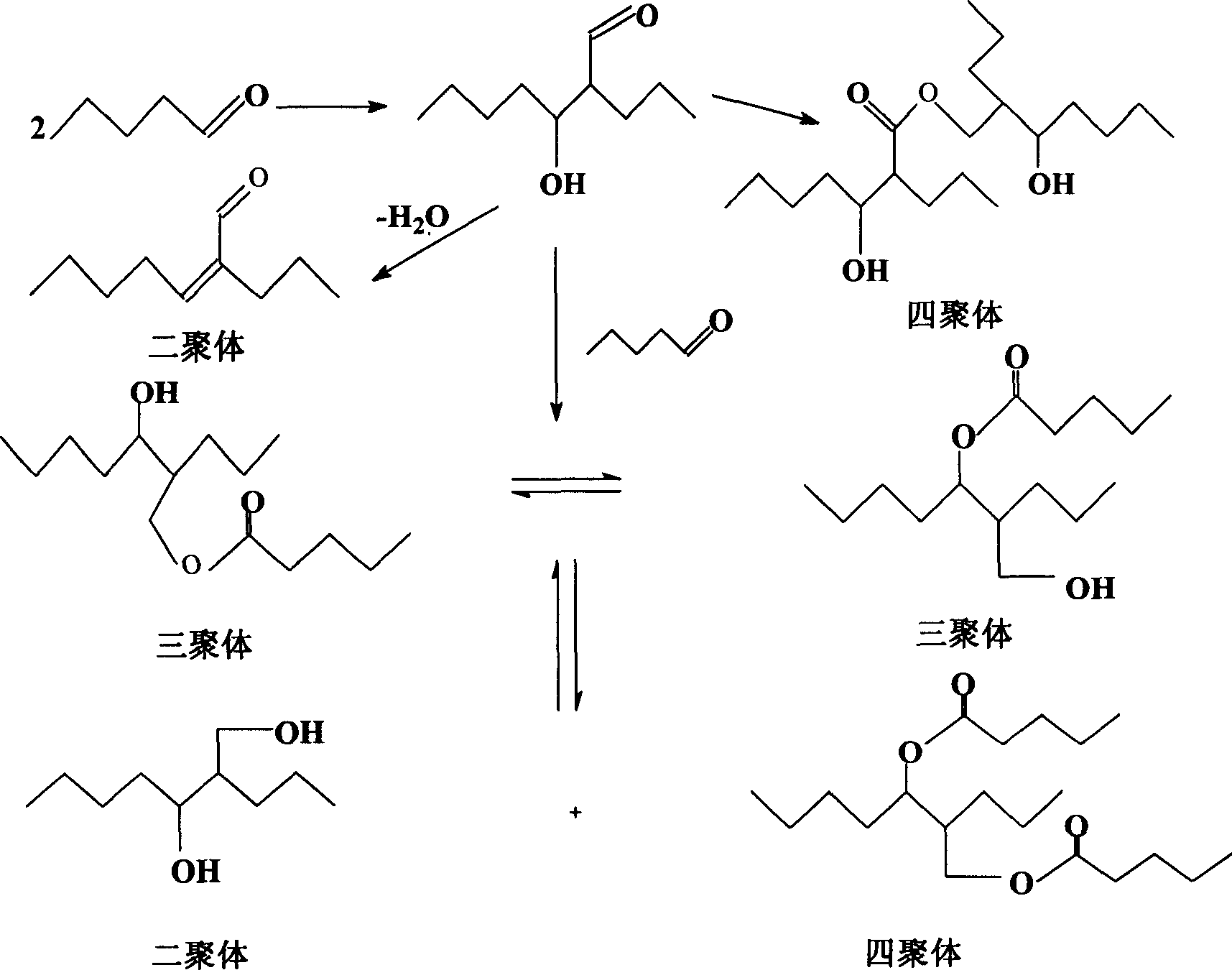
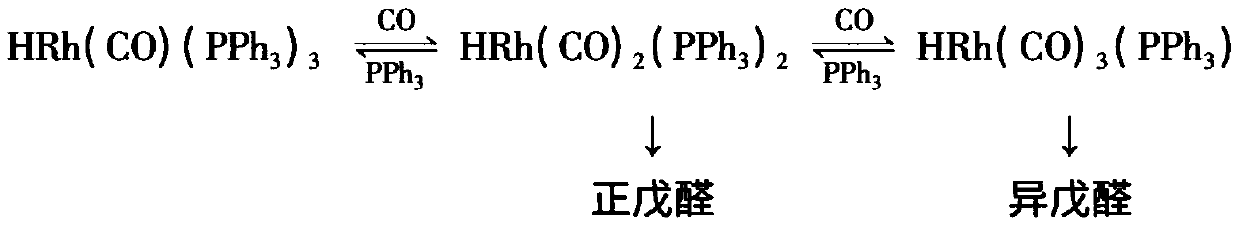
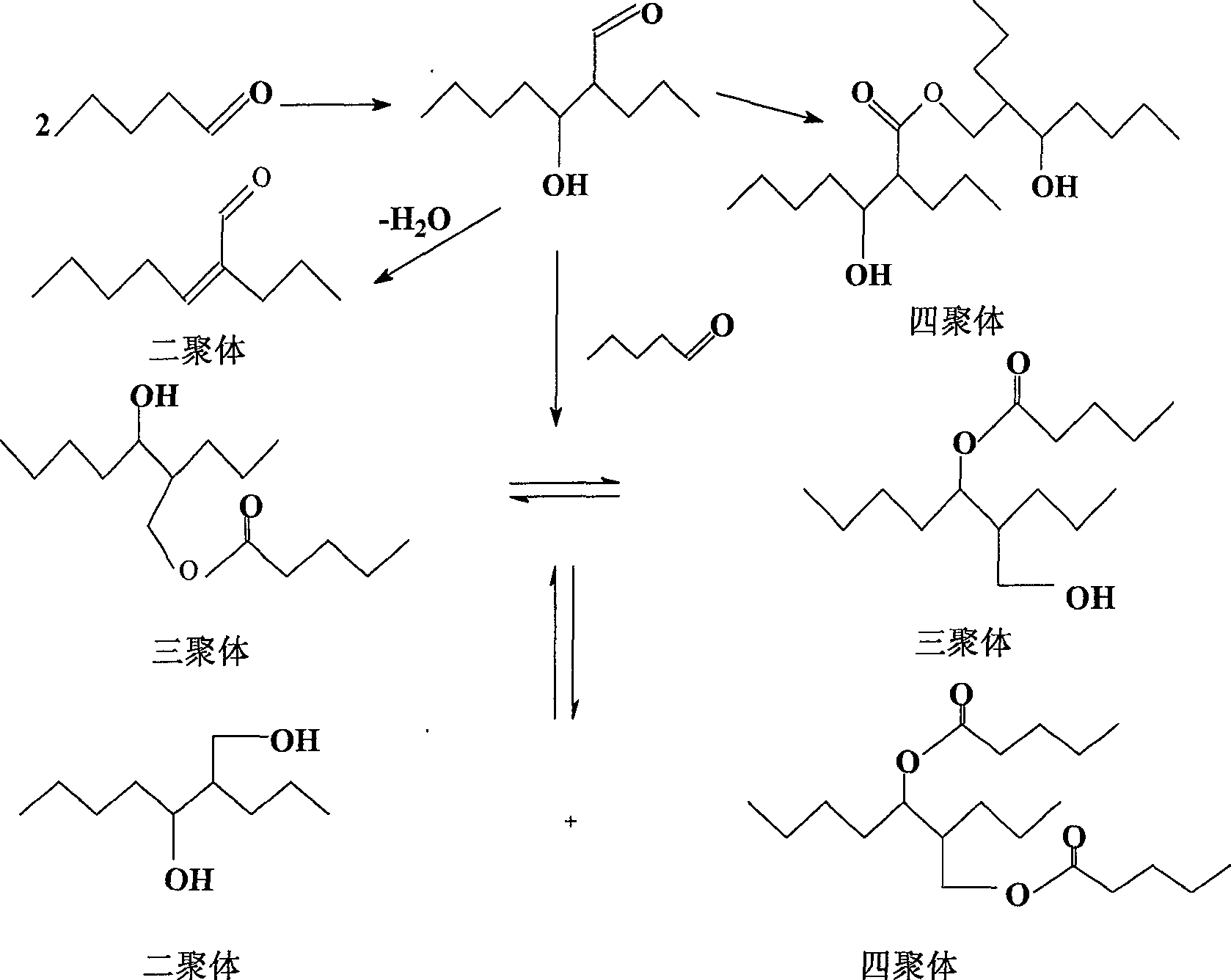
The invention discloses a process for continually preparing valeraldehyde, which reacts 1-butene raw material continually containing a little of trans / cis-2-butene, isobutene, butane with synthesis gas in the presence of the organic phosphinerhodium catalyst and triphenylphosphine. The process of the invention includes a reaction region, separation region and an activation region. Butene hydroformylation reaction liquid is evaporated, condensed and separated to two streams of raw product and circulating catalyst solution by film evaporation equipment, under shielding gas atmosphere of synthesis gas, and activated circulating catalyst solution enters reactor again. The present invention activates rhodium catalyst by catalyst activator, and controls or reduces acetal high boiling point byproducts which are generated by aldehyde polycondensation, enhance catalyst activity and regioselectivity of aldehyde. The film evaporation equipment used in the present invention is more effective than flash evaporator to separate catalyst solution and valeraldehyde producers in reaction liquor. Appropriate operational condition of film evaporation equipment can reduce or not generate aldehyde condensation, protive rhodium catalyst, enhance catalyst activity and prolong usage time of catalyst.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI +1
Method for preparing fatty aldehyde by means of a hydroformylation reaction
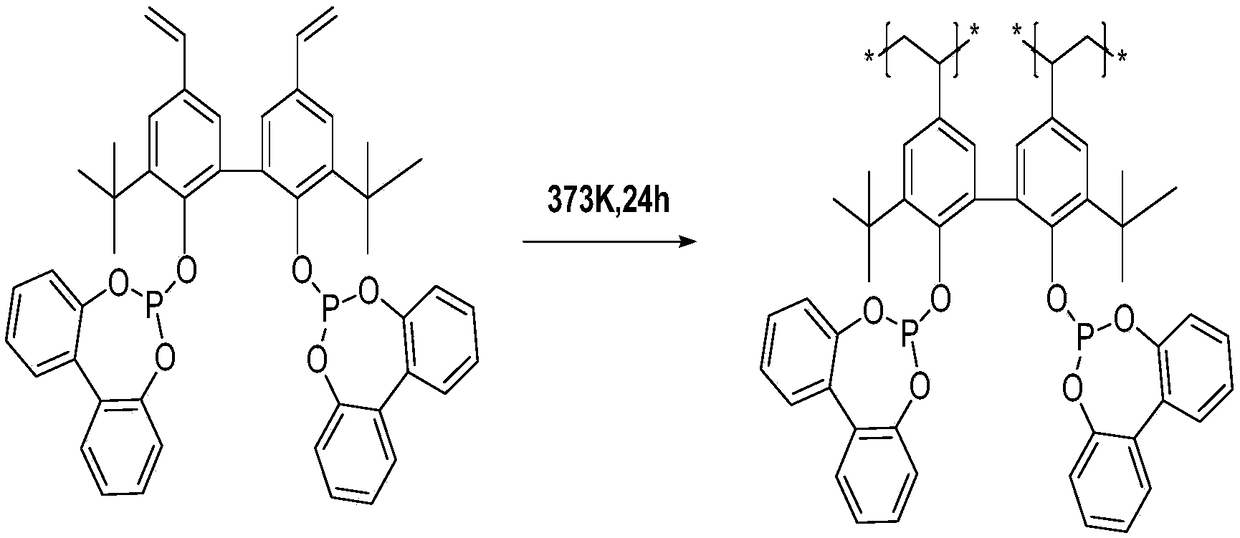
InactiveCN109456154ATake advantage ofGood choiceOrganic compound preparationPreparation by carbon monoxide reactionFormylation reactionDiphosphines
The invention discloses a method for preparing fatty aldehyde by means of a hydroformylation reaction. A catalyst system consisting of a water-soluble rhodium phosphine complex and a diphosphine ligand is used in water / organic two phases for catalyzing 1-butene, 2-butene and a mixture of the 1-butene to be subjected to the hydroformylation reaction so as to prepare n-valeraldehyde; due to the combination of the water-soluble bisphosphine ligand BISBIS and organic additives, the hydroformylation reaction of the butene is accelerated, and the molar ratio of the produced n-valeraldehyde / isovaleraldehyde is greater than 93 to 7. A catalyst aqueous solution is simple and convenient to separate from the product; furthermore, the catalytic performance is stable, the reaction conditions are mild,the service life of a catalyst is long, and the production cost of the n-valeraldehyde is remarkably lowered.
Owner:成都欣华源科技有限责任公司
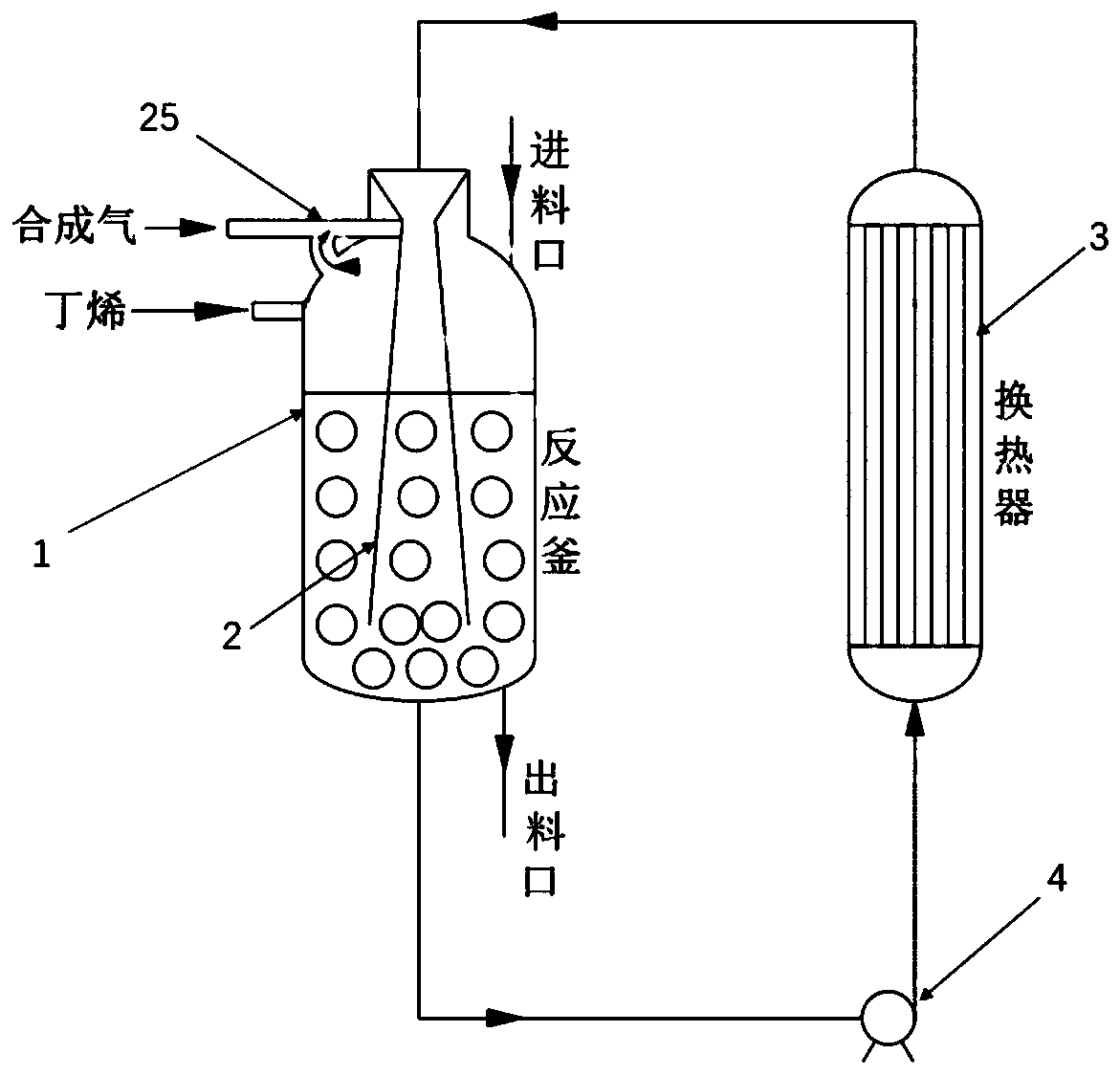
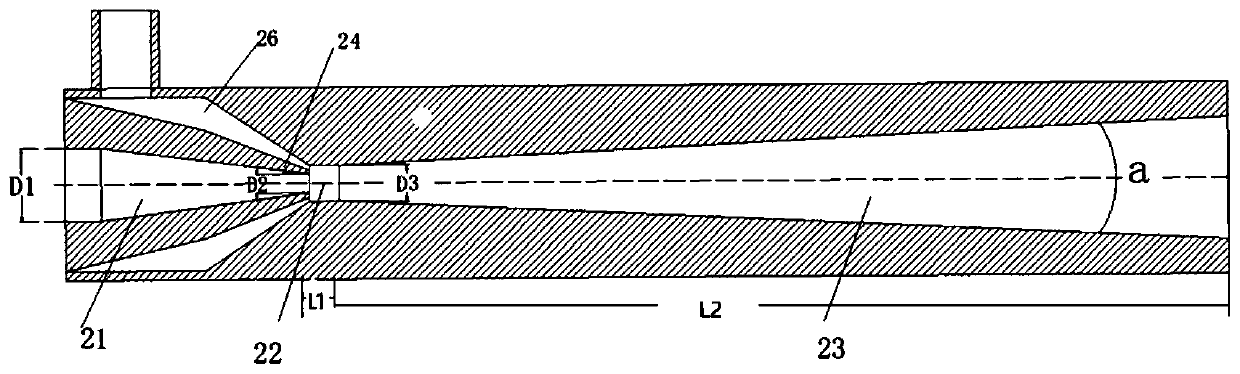
N-valeraldehyde preparation method and special venturi ejector
ActiveCN111217686AGood isotropic ratioReduce dosageFlow mixersOrganic compound preparationPtru catalystFormylation reaction
The invention discloses an n-valeraldehyde preparation method and a special venturi ejector, and the n-valeraldehyde preparation method adopts a loop reactor to prepare n-valeraldehyde through butenehydroformylation reaction, wherein the ratio of the inner diameter of the opening of the inlet section of the venturi ejector to the inner diameter of a nozzle to the inner diameter of a closing-in opening of an air chamber to the length of the mixing section to the length of the diffusion section in the loop reactor is 38: (1.5-5): (2-8): (10-50): (600-1700), and the opening angle of the diffusion section is 12-38 degrees. According to the n-valeraldehyde preparation method, butene, hydrogen and carbon monoxide are used as raw materials, toluene is used as a solvent, triphenylphosphine (TPP)and acetylacetonatodicarbonyl rhodium are used as catalysts, and the liquid linear speed at the nozzle of the venturi ejector is controlled to be 50-110 m / s in the reaction process. Through the designof a loop reactor, the normal-to-isomerism ratio of the valeraldehyde product can be effectively improved.
Owner:JIANGSU NUOMENG CHEM
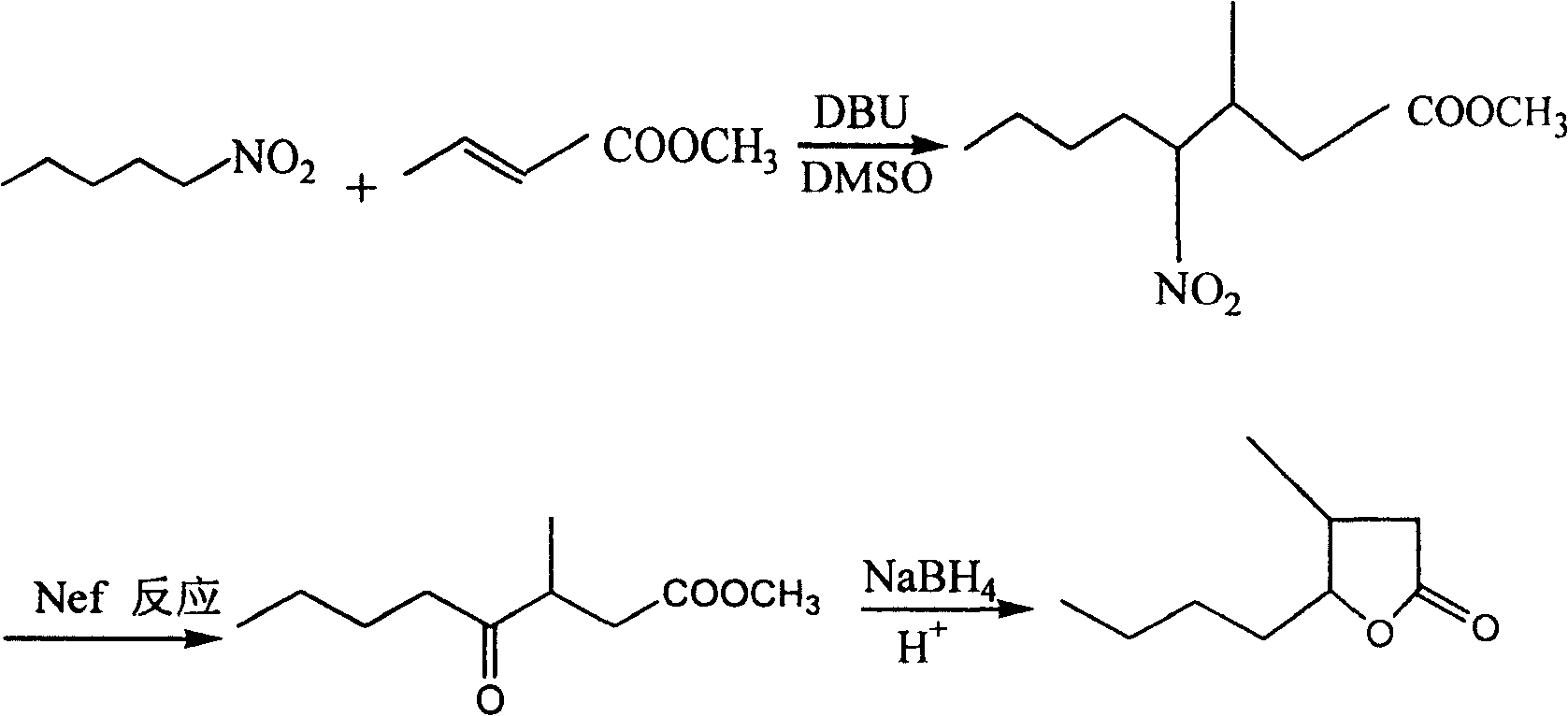
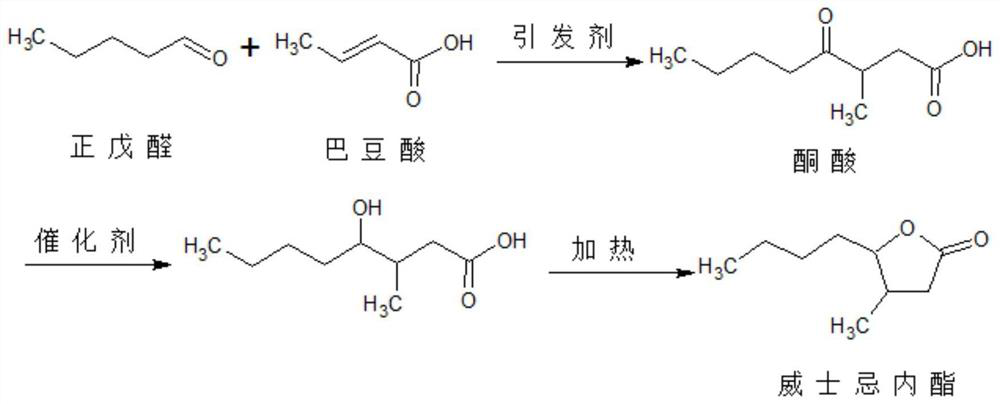
Method for preparing whisky lactone
ActiveCN1915984AEmission reductionAvoid it happening againOrganic chemistryKetonic acidsRefractive index
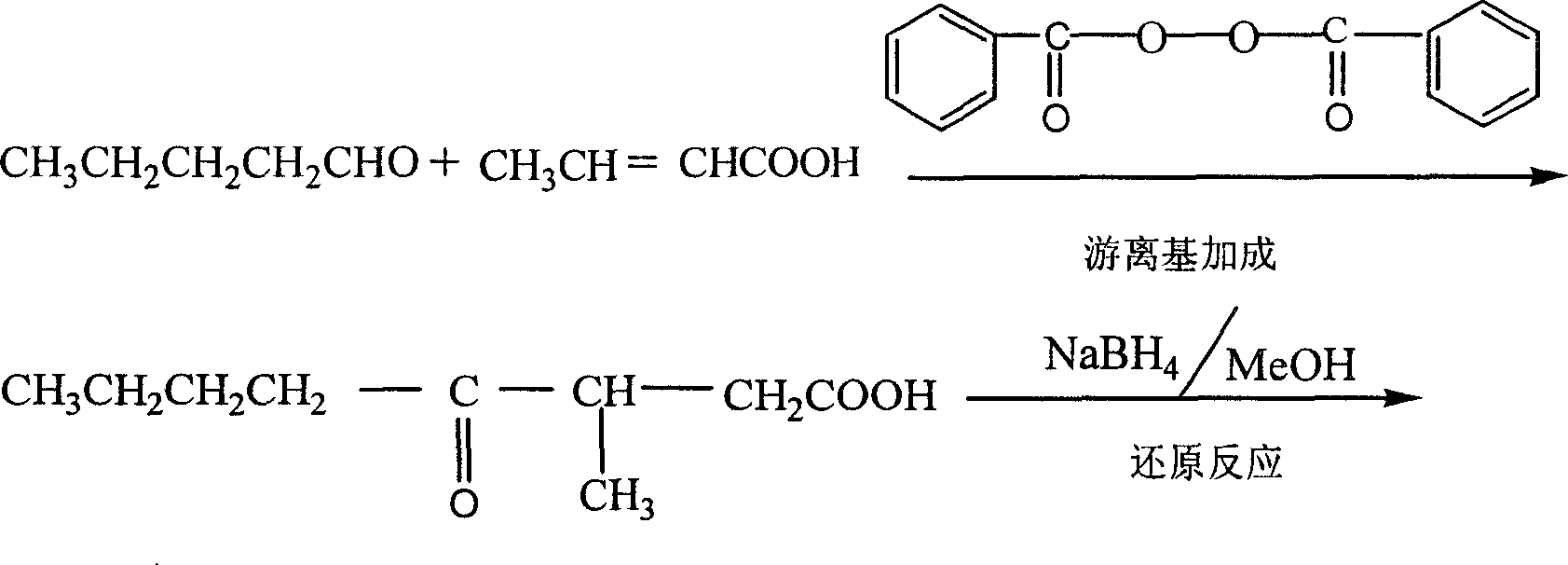
This invention provides a method for preparing whiskey lactone. The method comprises: (1) reacting n-valeraldehyde and crotonic acid by free radical addition under N2 protection in the presence of initiator and catalyst to obtain a mixture containing ketonic acid; (2) cooling, washing, drying and distilling to obtain ketonic acid; (3) hydrogenating in the presence of Raney Ni catalyst, filtering, heating for vaporizing, dehydrating and cyclizing to obtain whiskey lactone with a yield of 87% and a refractive index ND20 of 1.441-1.447. Determined by a combination of chromatography and mass spectrometry, the whiskey lactone comprises 98% cis-isomer, as well as 2% trans-isomer. The method has such advantages as high yield, simple process, reduced wastewater discharge and is good for environmental protection.
Owner:DALIAN LAIKE FINE CHEM CO LTD
Process method for one-step synthesis of long-chain alcohol by catalyzing aldehydes with solid catalyst
ActiveCN103708998AHigh activityImprove stabilityOrganic compound preparationPreparation by hydrogenationAlcoholHydrogen pressure
The invention relates to a process method for one-step synthesis of long-chain alcohol by catalyzing aldehydes with a solid catalyst. The method comprises the following steps of adding the solid catalyst and a raw material aldehyde into an autoclave, wherein an addition amount of the catalyst is 1-25% by mass that of the raw material aldehyde; reacting for 4-20 h at a temperature of 80-240 DEG C and under a hydrogen pressure of 0.5-8 MPa; and finally obtaining the long-chain alcohol. The raw material aldehyde is n-butyl aldehyde or n-valeraldehyde; the long-chain alcohol is octanol or decanol; the solid catalyst is a metal-solid acid (alkali) catalyst and comprises a metal, an auxiliary agent and a solid acid (alkali), wherein a mass percentage of the metal is the catalyst is 0.5-40%; the mass percentage of the auxiliary agent is 0-10%; and the balance being the solid acid (alkali). The provided environment-friendly novel process for the one-step synthesis of the long-chain alcohol by catalyzing the aldehydes with the solid catalyst can greatly shorten a process flow for synthesizing the long-chain alcohol and reduces equipment cost and operation cost.
Owner:HEBEI UNIV OF TECH
Preparation method for delta-decalactone
ActiveCN103058973ASimple methodSimple and fast operationOrganic chemistryBiochemical engineeringCombinatorial chemistry
The invention discloses a preparation method for delta-decalactone. The preparation method comprises the steps of serving cyclopentanone and n-valeraldehyde as original raw materials, simultaneously carrying out the two steps of aldol condensation and catalytic hydrogenation, synthesizing a 2-amyl cyclopentanone midbody by one-pot reaction, then carrying out oxidation ring enlargement, embedding an oxygen atom between keto and amyl, and synthesizing the delta-decalactone. The preparation method for the delta-decalactone is easy to do, simple and convenient to operate, and few in procedure. The product yield is improved by 3-4 percent compared with the method for preparation of the aldol condensation, the catalytic hydrogenation and oxidation step by step, only two steps of rectification are required, power consumption is reduced, and the preparation method for the delta-decalactone is applied to industrial production.
Owner:HUAIAN WAN BANG SPICE IND CO LTD
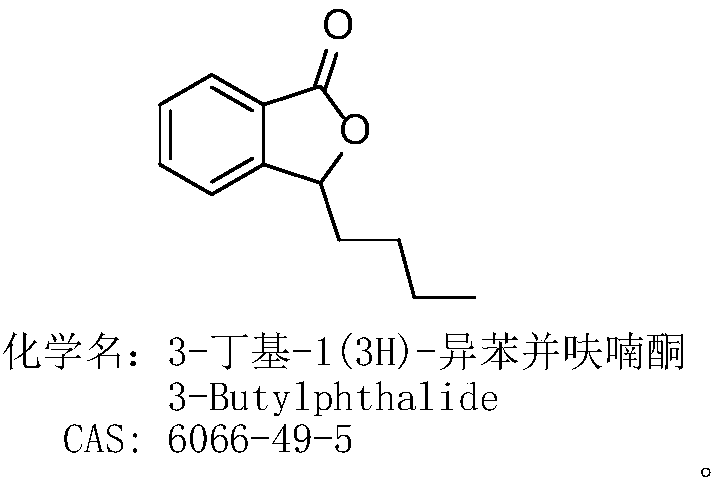
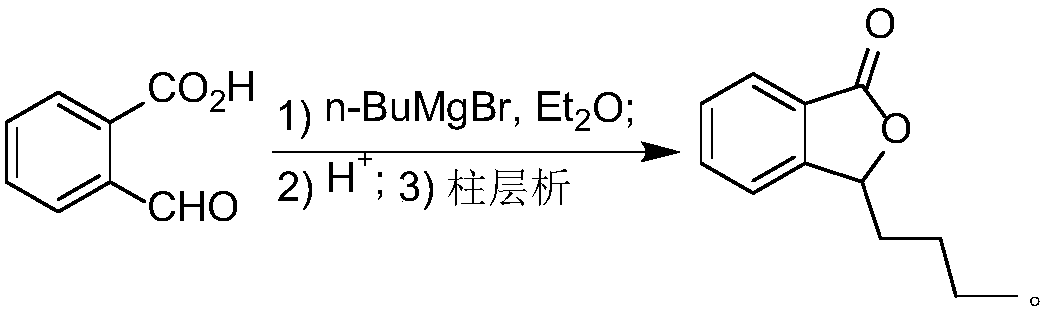
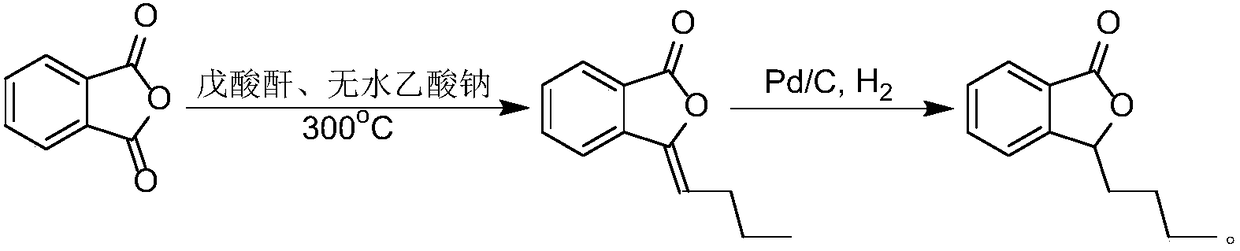
Process for preparing butyphthalide
ActiveCN108203421AEasy to operateAdvantages of industrial productionOrganic chemistryBenzeneButylphthalide
The invention relates to a process for preparing butyphthalide. A one-pot method is adopted, the process is easy to operate, and industrial production has remarkable advantages. The process comprisesthe following steps: making 1,2-dibromo-benzene serving as an initial material react with carbon dioxide ice under the action of n-BuLi to generate an intermediate; directly adding n-BuLi into an intermediate solution without quenching the reaction; making the mixture react with n-valeraldehyde; treating a reaction liquid with HCl; and performing column chromatography and purification to obtain the butylphthalide.
Owner:福建省宝诺医药研发有限公司
Method for one-step synthesis of 2-amyl-2-cyclopentenone
The invention provides a method for one-step synthesis of 2-amyl-2-cyclopentenone. The method is characterized by comprising the steps of dropwise adding a mixed solution of n-valeraldehyde and cyclopentanone to alkali liquor containing a composite catalyst in a mixed gas atmosphere, and synthesizing 2-amyl-2-cyclopentenone through one-step reaction, wherein the mixed gas is the mixed gas of nitrogen and hydrogen. According to the method, 2-amyl-2-cyclopentenone is synthesized through a one-step method, the total yield is high, the cost of raw materials and auxiliary materials is relatively low, the conversion rate of n-valeraldehyde is 99.4%-99.8%, the selectivity is 87.23%-90.68%, and the yield of 2-amyl-2-cyclopentenone is 87.05%-90.5%. The used composite catalyst can be applied for more than 25-40 times. According to the method, byproducts are few, the prepared product is high in purity, the generated wastewater is relatively less, and the environment is protected.
Owner:SHANDONG NHU PHARMA
Green technology for preparing n-valeric acid from low-purity n-valeraldehyde by air oxidation
InactiveCN106146287AReduce pollutionReduce processing costsOrganic compound preparationCarboxylic compound preparationReaction temperatureN-valeraldehyde
The invention discloses a green technology for preparing n-valeric acid from low-purity n-valeraldehyde by air oxidation. The green technology includes the steps: adding the low-purity n-valeraldehyde into a stirred bubbling tank reactor with outer circulation; bubbling into air or oxygen or oxygen-enriched air or oxygen-denuded air; decompressing, rectifying and purifying for products after oxidation reaction to obtain the n-valeric acid with purity of more than 98%. The content of the n-valeraldehyde is lower than 80%, air speed is 100-2000L / min, reaction temperature is 25-100 DEG C, reaction time is 1-30 hours, the conversion rate of the n-valeraldehyde reaches 97% or above, selectivity reaches 98% or above, single decompressing and distilling rate reaches 86% or above. By the aid of the green technology, the air serves as an oxidizing agent, any catalysts are omitted, and the green technology has the advantages of smooth reaction, high efficiency, low cost, green, environmental protection and the like and a wide application and market prospect.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY +1
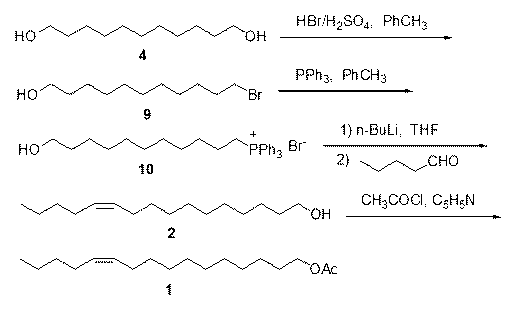
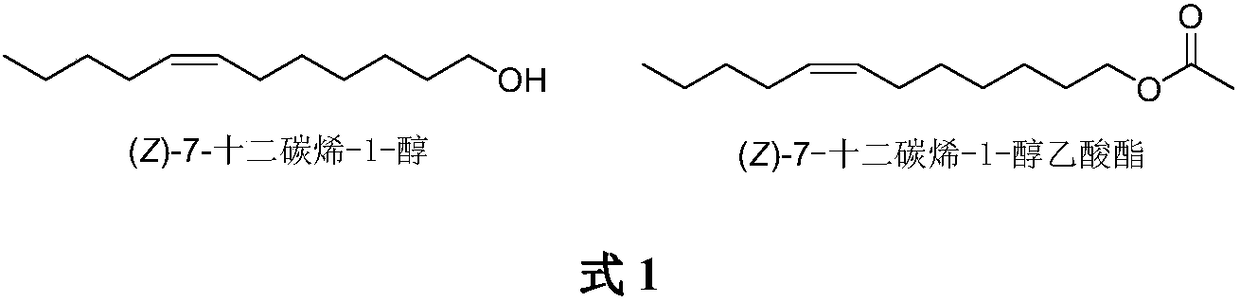
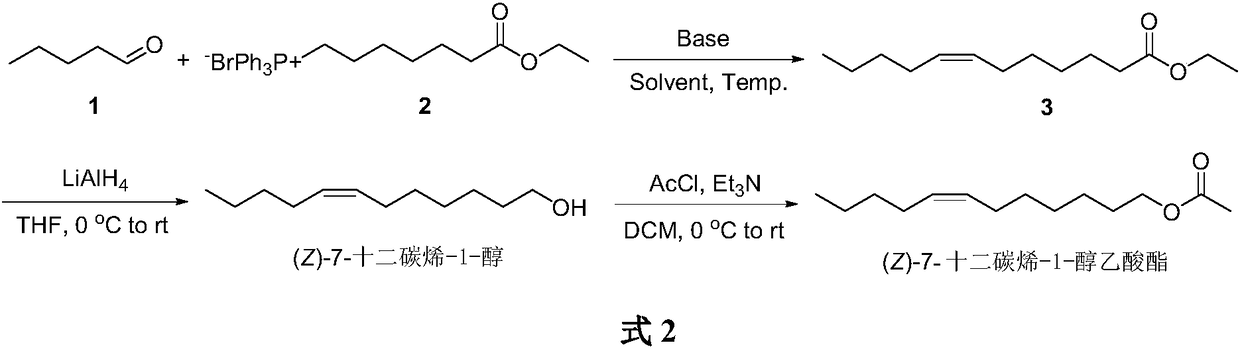
Synthesis of (Z)-7-dodecylene-1-alcohol and acetic ester thereof
The invention belongs to the technical field of insect pheromone synthesis, and discloses a novel method for synthesizing (Z)-7-dodecylene-1-alcohol and acetic ester thereof. The method comprises thefollowing steps: taking n-valeraldehyde as a starting material and reacting with a Wittig reagent to obtain (Z)-7-dodecenoic acid ethyl ester; then performing reduction with lithium aluminum hydroxideto obtain (Z)-dodeca-carbon-7-alkene-1-alcohol; finally taking (Z)-dodeca-carbon-7-alkene-1-alcohol to react with acetyl chloride to obtain (Z)-7-dodecylene-1-farnesyl acetate. By adopting the method, Z-shaped double bonds are directly constructed through a coupling reaction of the Wittig reagent carrying an ester group at the tail end with aldehyde. The method is simple in synthesis route, and is environmentally friendly.
Owner:CHINA AGRI UNIV
Method of conducting hydroformylation reaction on C4 mixture to prepare aldehydes
PendingCN110862307AAvoid separationPreparation by carbon monoxide reactionChemical recyclingPolymer sciencePtru catalyst
Provided is a method of conducting a hydroformylation reaction on a C4 mixture to prepare aldehydes. The method includes the steps: S1, in the presence of a first catalyst, the C4 mixture and a syngasmake contact in a first reaction region to be subjected to the hydroformylation reaction, and a first reaction product containing n-valeraldehyde and 2-methylbutyraldehyde is obtained, wherein the C4mixture is composed of 1-butylene and isobutylene and obtained by removing 2-butylene and 1,3-butadiene; S2, separation is conducted on the first reaction product, and a mixture of n-valeraldehyde and 2-methylbutyraldehyde, unreacted raw materials and a material flow which contains the first catalyst are obtained; and S3, in the presence of a second catalyst, the unreacted raw materials and the syngas make contact in a second reaction region to be subjected to the hydroformylation reaction, a second reaction product containing 3-methylbutyraldehyde is obtained, and optionally, the second reaction product is purified. According to the method of conducting the hydroformylation reaction on the C4 mixture to prepare the aldehydes, in a staged reaction mode, 1-butylene and isobutylene are subjected to the hydroformylation reaction separately to obtain different products, and advance separation of 1-butylene and isobutylene is avoided.
Owner:CHINA PETROLEUM & CHEM CORP +1
A kind of method of synthesizing 2-pentyl-2-cyclopentenone in one step
ActiveCN106699528BLow costHigh yieldCarbonyl compound preparation by condensationNitrogen gasMixed gas
The invention provides a method for one-step synthesis of 2-amyl-2-cyclopentenone. The method is characterized by comprising the steps of dropwise adding a mixed solution of n-valeraldehyde and cyclopentanone to alkali liquor containing a composite catalyst in a mixed gas atmosphere, and synthesizing 2-amyl-2-cyclopentenone through one-step reaction, wherein the mixed gas is the mixed gas of nitrogen and hydrogen. According to the method, 2-amyl-2-cyclopentenone is synthesized through a one-step method, the total yield is high, the cost of raw materials and auxiliary materials is relatively low, the conversion rate of n-valeraldehyde is 99.4%-99.8%, the selectivity is 87.23%-90.68%, and the yield of 2-amyl-2-cyclopentenone is 87.05%-90.5%. The used composite catalyst can be applied for more than 25-40 times. According to the method, byproducts are few, the prepared product is high in purity, the generated wastewater is relatively less, and the environment is protected.
Owner:SHANDONG NHU PHARMA
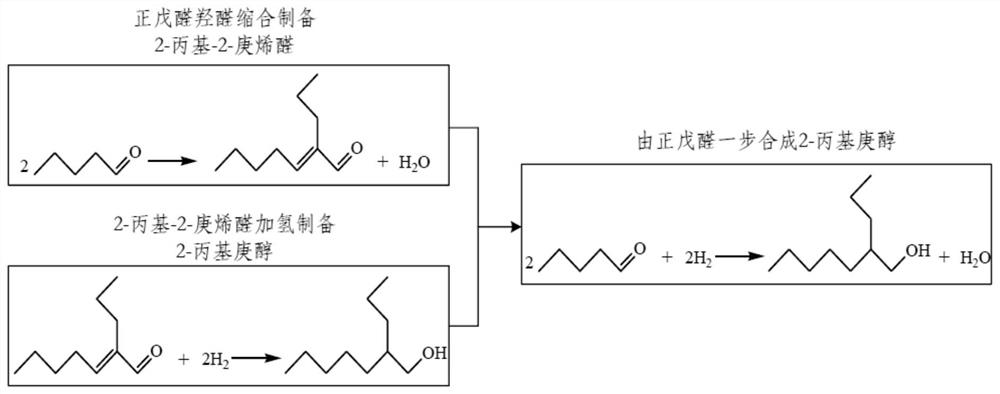
Supported metal-acidic ionic liquid catalyst and application thereof
ActiveCN110075916AHigh industrial application valueGood choiceOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsN-valeraldehydeIonic liquid
The invention provides a supported metal-acidic ionic liquid catalyst which is supported with reductive metal and acidic ionic liquid simultaneously on a solid phase carrier. The catalyst provided bythe invention has advantages of high catalytic activity and selectivity, easy separation and the like, and is especially suitable for technical requirements on direct synthesis of 2-propylheptanol from n-valeraldehyde. A green novel process for directly synthesizing 2-propylheptanol from n-valeraldehyde catalyzed by supported metal-acidic ionic liquid, provided by the invention, can greatly shorten process flows of industrial synthesis of 2-propylheptanol and reduce equipment costs and operation costs.
Owner:HEBEI UNIV OF TECH +1
Preparation method for delta-decalactone
ActiveCN103058973BSimple methodSimple and fast operationOrganic chemistryBiochemical engineeringCombinatorial chemistry
The invention discloses a preparation method for delta-decalactone. The preparation method comprises the steps of serving cyclopentanone and n-valeraldehyde as original raw materials, simultaneously carrying out the two steps of aldol condensation and catalytic hydrogenation, synthesizing a 2-amyl cyclopentanone midbody by one-pot reaction, then carrying out oxidation ring enlargement, embedding an oxygen atom between keto and amyl, and synthesizing the delta-decalactone. The preparation method for the delta-decalactone is easy to do, simple and convenient to operate, and few in procedure. The product yield is improved by 3-4 percent compared with the method for preparation of the aldol condensation, the catalytic hydrogenation and oxidation step by step, only two steps of rectification are required, power consumption is reduced, and the preparation method for the delta-decalactone is applied to industrial production.
Owner:HUAIAN WAN BANG SPICE IND CO LTD
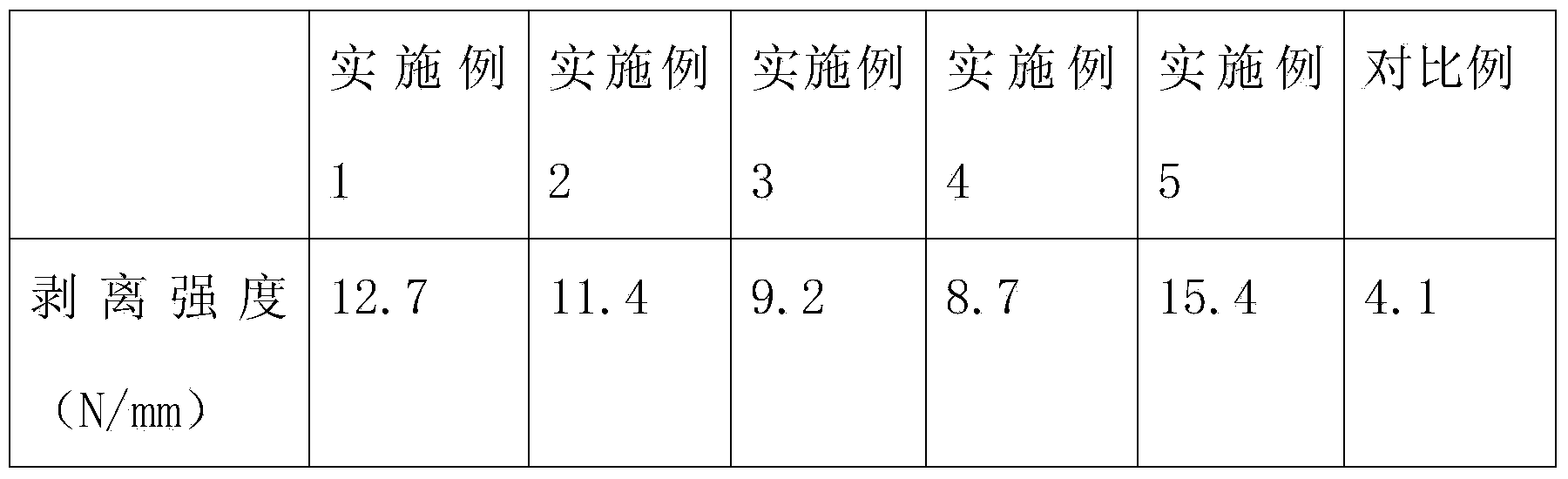
High-temperature-resistant wood floor nano adhesive and preparation method thereof
ActiveCN104277748AImprove high temperature resistanceHigh peel strengthNon-macromolecular adhesive additivesAldehyde/ketone condensation polymer adhesivesAdhesiveSilanes
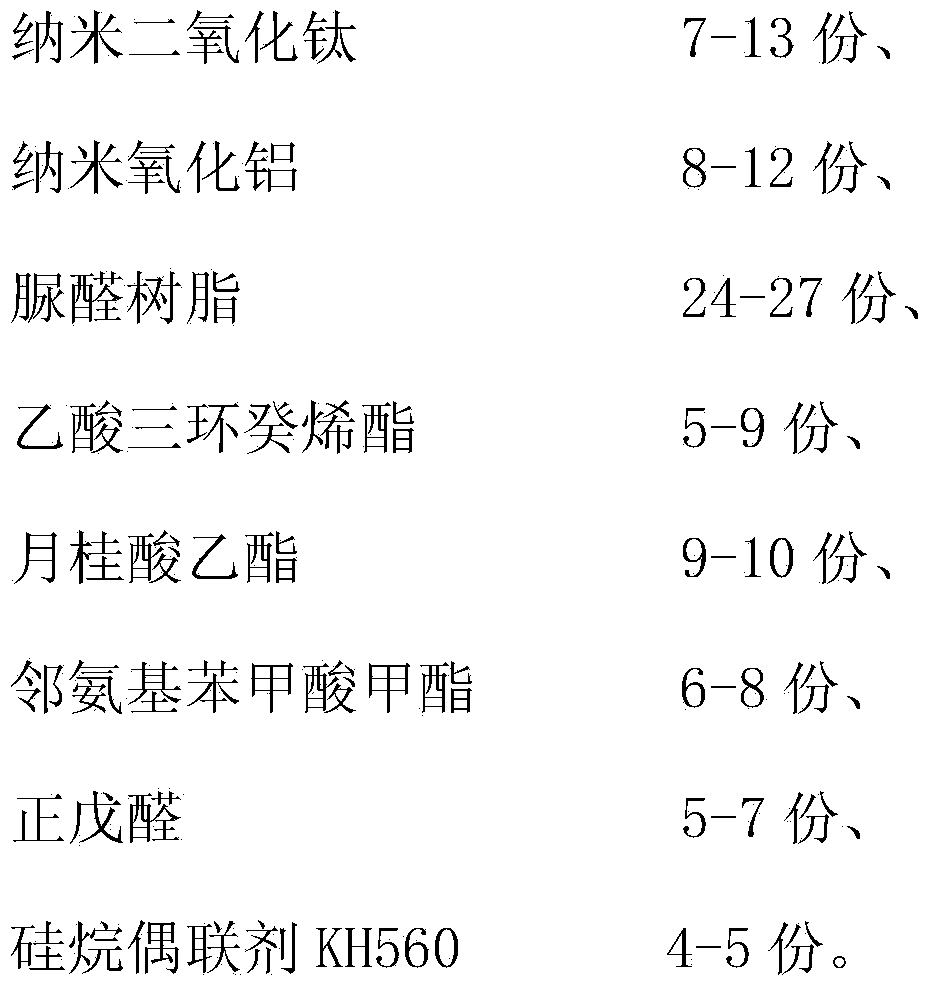
The invention belongs to the field of the adhesives and discloses a high-temperature-resistant wood floor nano adhesive and a preparation method thereof. The nano adhesive is prepared from nano titanium dioxide, nano aluminium oxide, urea resin, verdyl acetate, ethyl laurate, methyl anthranilate, n-valeraldehyde and a silane coupling agent KH560. The preparation method comprises the following steps: (1) evenly mixing the two nano compounds, namely nano titanium dioxide and nano aluminium oxide, thereby obtaining powder A; (2) adding the urea resin, verdyl acetate, ethyl laurate and methyl anthranilate while stirring mechanically; (3) heating the mixture of the step (2) and then adding valeraldehyde, the silane coupling agent KH560 and the powder A prepared in the step (1), and mixing and stirring evenly to obtain the high temperature-resistant wood floor nano adhesive.
Owner:封开县威利邦木业有限公司
Method for preparing whisky lactone
ActiveCN100413856CEmission reductionAvoid it happening againOrganic chemistryKetonic acidsRefractive index
This invention provides a method for preparing whiskey lactone. The method comprises: (1) reacting n-valeraldehyde and crotonic acid by free radical addition under N2 protection in the presence of initiator and catalyst to obtain a mixture containing ketonic acid; (2) cooling, washing, drying and distilling to obtain ketonic acid; (3) hydrogenating in the presence of Raney Ni catalyst, filtering, heating for vaporizing, dehydrating and cyclizing to obtain whiskey lactone with a yield of 87% and a refractive index ND20 of 1.441-1.447. Determined by a combination of chromatography and mass spectrometry, the whiskey lactone comprises 98% cis-isomer, as well as 2% trans-isomer. The method has such advantages as high yield, simple process, reduced wastewater discharge and is good for environmental protection.
Owner:DALIAN LAIKE FINE CHEM CO LTD
Method for preparing amyl alcohol by utilizing n-valeraldehyde
InactiveCN105884575AReduce energy consumptionReduce manufacturing costMolecular sieve catalystsOrganic compound preparationManufacturing cost reductionSorbent
The invention discloses a method for preparing amyl alcohol by utilizing n-valeraldehyde. The method comprises the following steps: 1) adding n-valeraldehyde and a hydrogenation catalyst into a high-pressure kettle, filling and sealing; 2) replacing for five years by use of 1-2MPa hydrogen gas, charging 5 MPa hydrogen gas into the kettle, heating to 200 DEG C, preserving the heat to react for 4 hours, cooling, releasing pressure, opening the kettle and filtering after the reaction is ended, getting upper-layer organic phase, thereby obtaining a semi-finished product amyl alcohol; 3) adding sodium hydroxide into the semi-finished product amyl alcohol, shaking, stewing and layering, discarding a water layer, filtering an organic layer, and keeping filtrate; and 4) introducing the filtrate obtained in the step 3) into an adsorbent column with aluminum oxide to carry out adsorption treatment. According to the technical scheme adopted by the invention, a n-valeraldehyde conversion rate can be up to 99.8%, the purity of the obtained amyl alcohol can be up to 99.99%, the energy consumption is low, the manufacturing cost can be reduced, corrosion on equipment is avoided, the pollution is less and the safety is high on the premise of guaranteeing a conversion rate and purity.
Owner:SHANDONG CHENGTAI CHEM IND
Illiberis ulmivora Graeser sex attractant and preparation method thereof
ActiveCN113040145ARaw materials are easy to getSimple and fast operationBiocidePreparation from carboxylic acid halidesALUMINUM HYDRIDETetradecenoic Acid
The invention provides an Illiberis ulmivora Graeser sex attractant, which comprises cis-7-dodecenoic acid-2-butyl ester and cis-9-tetradecenoic acid-2-butyl ester. The invention also provides a preparation method of the Illiberis ulmivora Graeser sex attractant, which comprises the steps of reducing pimelic acid and azelaic acid into 7-hydroxyheptanoic acid through lithium aluminum hydride, brominating the 7-hydroxyheptanoic acid, performing acylating chlorination on the 7-hydroxyheptanoic acid and thionyl chloride, esterifying the 7-hydroxyheptanoic acid and the thionyl chloride with sec-butyl alcohol to form 7-bromoheptanoic acid-2-butanol ester and 9-bromononanoic acid-2-butanol ester, preparing corresponding phosphine salt from the 7-bromoheptanoic acid-2-butanol ester and the 9-bromononanoic acid-2-butanol ester and triphenylphosphine, and then carrying out wittig reaction with n-valeraldehyde to synthesize a target compound. According to the Illiberis ulmivora Graeser sex attractant and the preparation method thereof, raw materials are easy to obtain, operation is easy and convenient, the cost is low, the product yield is high, the technological process is short, synthesis reaction conditions are mild, no danger exists, the product is easy to separate, for the stereoisomerization problem, unstable phosphorus ylide reacts with fatty aldehyde, the product is mainly cis-olefin, the yield is 90% or above, the stable phosphorus ylide reacts with fatty aldehyde, and the product is mainly trans-olefin.
Owner:虫捕头(苏州)生物科技有限公司 +3
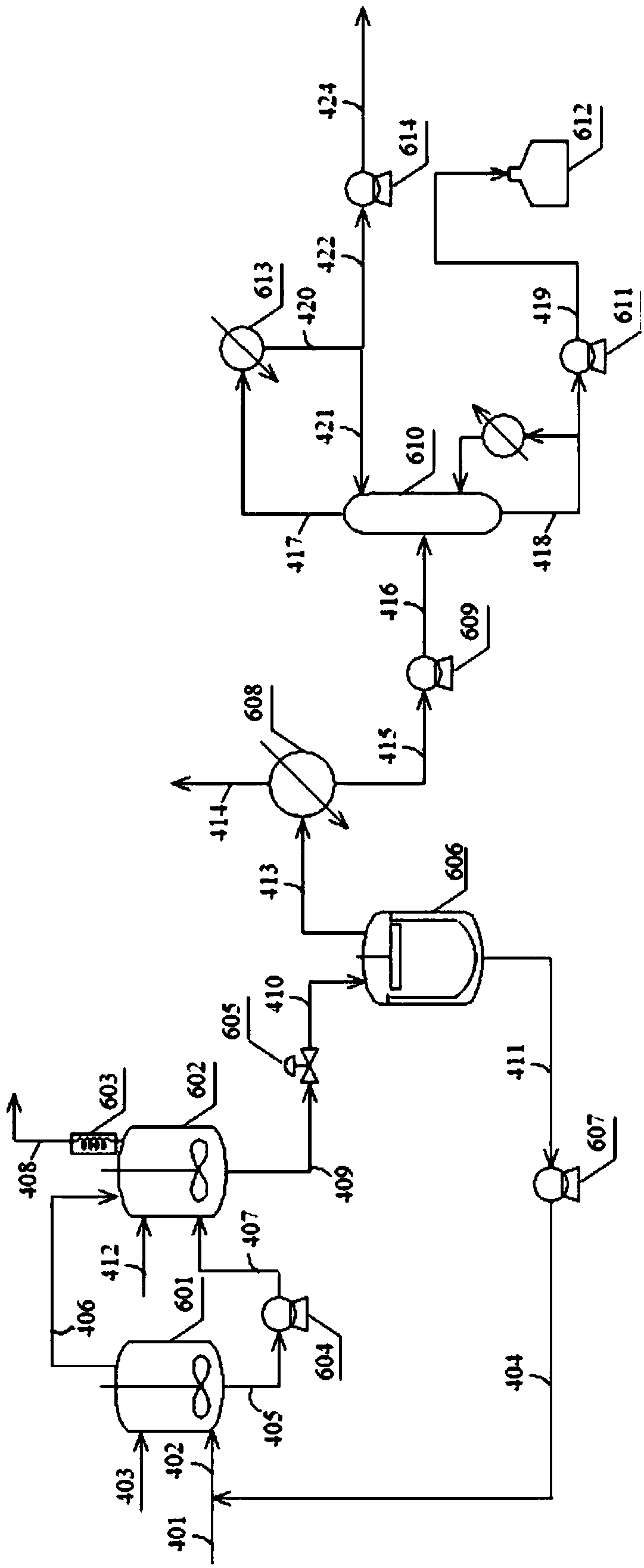
Process of continuously preparing n-pentanal
ActiveCN100526279CHigh activityHigh regional selectivityPreparation by carbon monoxide reactionFormylation reactionBiological activation
The invention discloses a process for continuously preparing n-valeraldehyde. The process is a continuous reaction of 1-butene raw material containing a small amount of cis / trans-2-butene, isobutene and butane with synthesis gas in the presence of organophosphine carbonyl rhodium catalyst and triphenylphosphine to prepare n-valeraldehyde . The technological process of the present invention includes a reaction zone, a separation zone and an activation zone. In the flow reaction device, the butene hydroformylation reaction liquid is evaporated and condensed under the protective atmosphere of synthesis gas by thin film evaporation equipment, and separated into two streams: the crude product and the circulating catalyst solution. The circulating catalyst is activated and then enters reactor. The invention adopts a catalyst activator, activates the rhodium catalyst, controls or reduces the acetal by-product with high boiling point generated in the aldehyde polycondensation reaction, improves the activity of the catalyst, and improves the regioselectivity of the aldehyde. The film evaporation equipment selected by the present invention is more effective than the flash evaporator in separating the catalyst solution and the valeraldehyde product in the reaction liquid. Appropriate operating conditions of the film evaporator can reduce or not produce aldehyde condensation, protect the rhodium catalyst, improve the catalyst activity and prolong the service life of the catalyst.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI +1
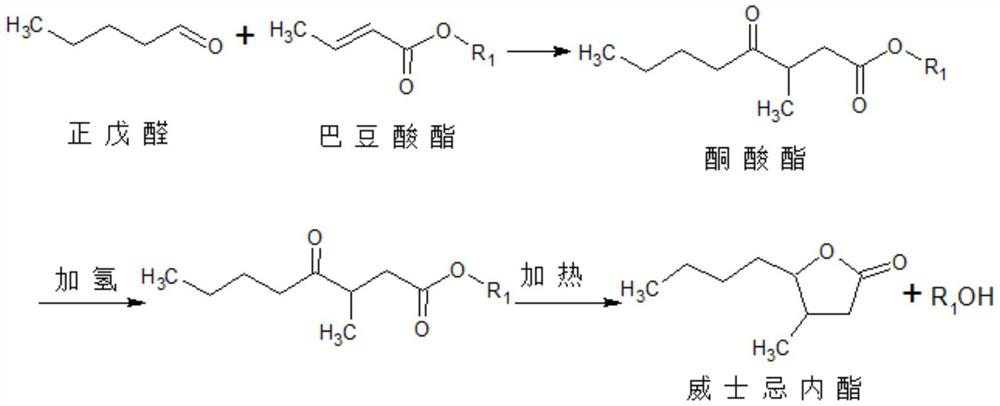
A kind of synthesis technique of whiskey lactone
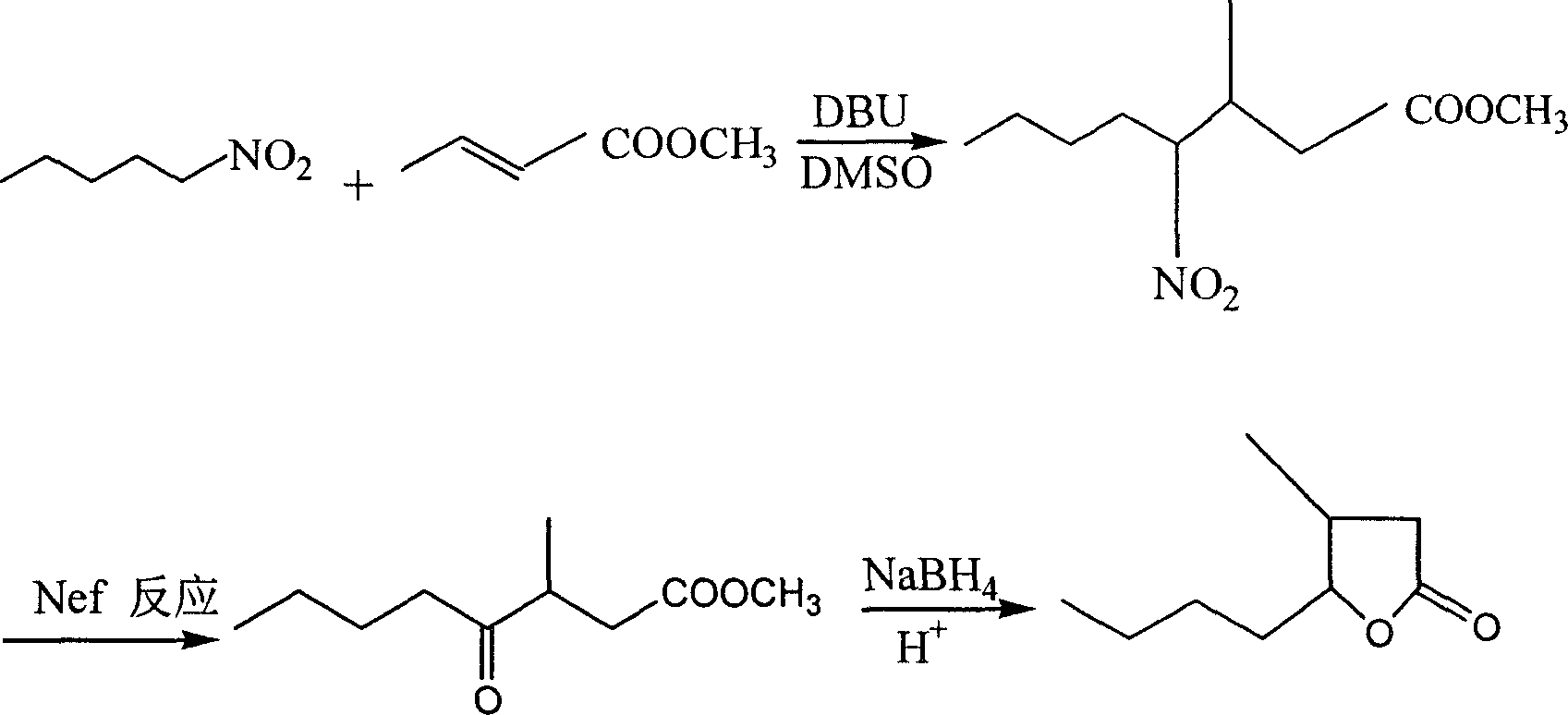
ActiveCN110437181BAvoid stabilityAvoiding problems that reduce n-valeraldehyde conversionOrganic chemistryAlcoholOrganic synthesis
The invention discloses a synthesis process of whiskey lactone, which relates to the technical field of organic synthesis. It uses n-valeraldehyde and crotonate as reaction raw materials and alcohol as a reaction solvent to prepare a ketoester through an addition reaction, and then reacts with a catalyst Whiskey lactone can be obtained through hydrogenation reaction under the action; the present invention uses n-valeraldehyde and crotonate as reaction raw materials, and can prepare ketoester without initiator and cocatalyst, avoiding the problem of using acidic medium as cocatalyst. Problems with reduced n-valeraldehyde conversion due to n-valeraldehyde instability.
Owner:ANHUI HYEA AROMAS
A kind of high temperature resistant wooden floor nano-adhesive and preparation method thereof
ActiveCN104277748BImprove high temperature resistanceHigh peel strengthNon-macromolecular adhesive additivesAldehyde/ketone condensation polymer adhesivesSilanesAdhesive
The invention belongs to the field of the adhesives and discloses a high-temperature-resistant wood floor nano adhesive and a preparation method thereof. The nano adhesive is prepared from nano titanium dioxide, nano aluminium oxide, urea resin, verdyl acetate, ethyl laurate, methyl anthranilate, n-valeraldehyde and a silane coupling agent KH560. The preparation method comprises the following steps: (1) evenly mixing the two nano compounds, namely nano titanium dioxide and nano aluminium oxide, thereby obtaining powder A; (2) adding the urea resin, verdyl acetate, ethyl laurate and methyl anthranilate while stirring mechanically; (3) heating the mixture of the step (2) and then adding valeraldehyde, the silane coupling agent KH560 and the powder A prepared in the step (1), and mixing and stirring evenly to obtain the high temperature-resistant wood floor nano adhesive.
Owner:封开县威利邦木业有限公司
A kind of supported metal-acidic ionic liquid catalyst and application thereof
ActiveCN110075916BImprove catalytic performanceHigh industrial application valueOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystPhysical chemistry
The invention provides a supported metal-acidic ionic liquid catalyst which is supported with reductive metal and acidic ionic liquid simultaneously on a solid phase carrier. The catalyst provided bythe invention has advantages of high catalytic activity and selectivity, easy separation and the like, and is especially suitable for technical requirements on direct synthesis of 2-propylheptanol from n-valeraldehyde. A green novel process for directly synthesizing 2-propylheptanol from n-valeraldehyde catalyzed by supported metal-acidic ionic liquid, provided by the invention, can greatly shorten process flows of industrial synthesis of 2-propylheptanol and reduce equipment costs and operation costs.
Owner:HEBEI UNIV OF TECH +1
Acid-base double-site catalyst for carbon-carbon coupling reaction and application thereof
ActiveCN113941362AHigh catalytic activityGood choiceMolecular sieve catalystsOrganic compound preparationCyclohexanonePtru catalyst
The invention discloses an acid-base double-site catalyst for a carbon-carbon coupling reaction, the acid-base double-site catalyst is formed by compounding a catalyst A and a catalyst B, the catalyst A is selected from a metal oxide with a surface alkaline site, and the catalyst B is selected from a molecular sieve with a surface acidic site. The acid-base double-site catalyst is suitable for a carbon-carbon coupling reaction of one or more of an alcohol organic compound, an aldehyde organic compound, a ketone organic compound and an ester organic compound, the reaction rate of the carbon-carbon coupling reaction can be greatly improved, and the conversion rate of a substrate and the yield and selectivity of the target product are remarkably improved. The catalyst especially has excellent catalytic activity reaction for co-production of mesityl oxide and isophorone through separate condensation coupling of acetone and self-condensation coupling of cyclohexanone to produce o-cyclohexenyl cyclohexanone, the reaction for preparing methyl methacrylate through condensation coupling of methyl propionate and formaldehyde and the reaction for preparing pentylidene cyclopentanone through condensation coupling of cyclopentanone and n-valeraldehyde.
Owner:ZHEJIANG UNIV
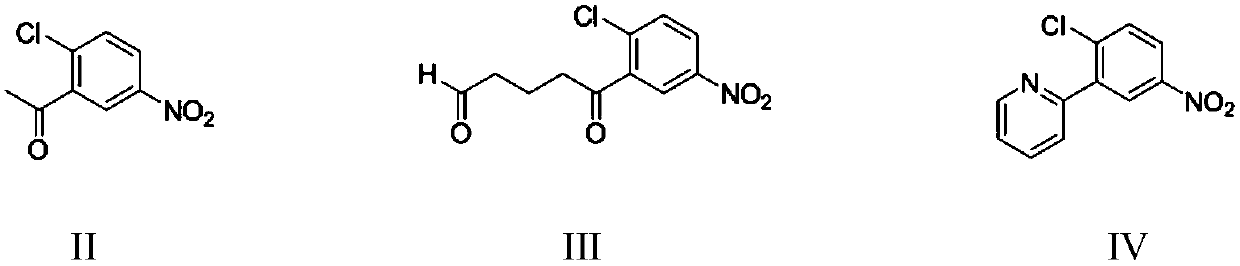
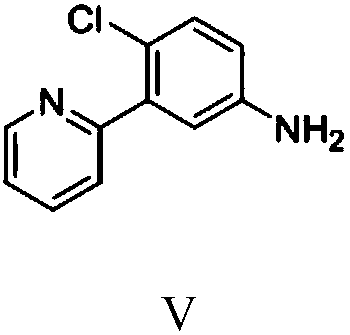
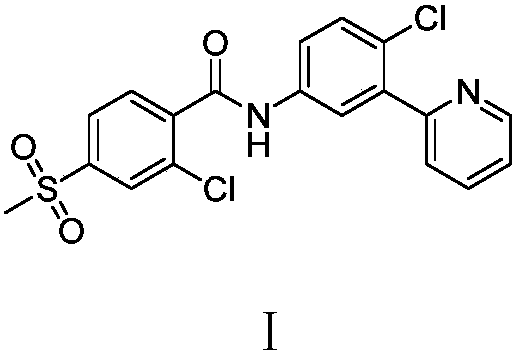
Preparation method of vimodegil
The invention provides a preparation method of vimodegil. 2-chloro-5-nitroacetophenone is used as a raw material; 5-oxo-5-(2-chloro-5-nitrophenyl) n-valeraldehyde is prepared through an addition reaction between 2-chloro-5-nitroacetophenone and acrolein, then 5-oxo-5-(2-chloro-5-nitrophenyl) n-valeraldehyde reacts with ammonia and an oxidizing agent in sequence to prepare 2-(2-chloro-5-nitrophenyl) pyridine, 2-(2-chloro-5-nitrophenyl) pyridine is reduced to obtain 2-(2-chloro-5-aminophenyl) pyridine, and 2-(2-chloro-5-aminophenyl) pyridine reacts with 2-chloro-4-methylsulfonylbenzoyl chlorideto obtain vimodegil. The preparation method has the advantages of cheap and easily available raw materials, low cost, short technical process, safe, simple and convenient operation, easily controllable and easily realized reaction conditions, little amount of generated waste liquid, green, environmental friendliness, stable intermediate compounds, high reaction selectivity, high product purity, and high yield, and is suitable for industrial production.
Owner:XINFA PHARMA
Sweet and fragrant fructus momordicae essence and preparation process thereof
InactiveCN108690721AGreat tasteImprove anti-corrosion performanceEssential-oils/perfumesFlavorBenzaldehyde
The invention discloses sweet and fragrant fructus momordicae essence and a preparation process thereof. The essence is characterized by being prepared from the following preparation materials in percentage by weight: 0.2 percent of isovaleraldehyde, 0.1 percent of 2-methylbutyraldehyde, 0.1 percent of n-valeraldehyde, 0.13 percent of acetaldehyde, 0.5 percent of furfural, 0.2 percent of 5-methylfurfural, 0.05 percent of benzaldehyde, 0.3 percent of vanillin, 88.42 percent of propylene glycol and 10 percent of momordica grosvenori extraction liquid. Through the process and formula optimization, the sweet and fragrant ingredients of natural fructus momordicae are extracted; the medicine taste is reduced; the mouthfeel is improved, so that fructus momordicae extracts have the greater application range. Meanwhile, sodium benzoate is introduced; the anticorrosion performance of the fructus momordicae liquid is improved. Meanwhile, synthesis spice is added; the initial fragrance is regulated; the fragrance of the essence is stabilized; the quality of a finished product of a product is improved.
Owner:SHANGHAI LVZHOUYUAN PERFUME CO LTD
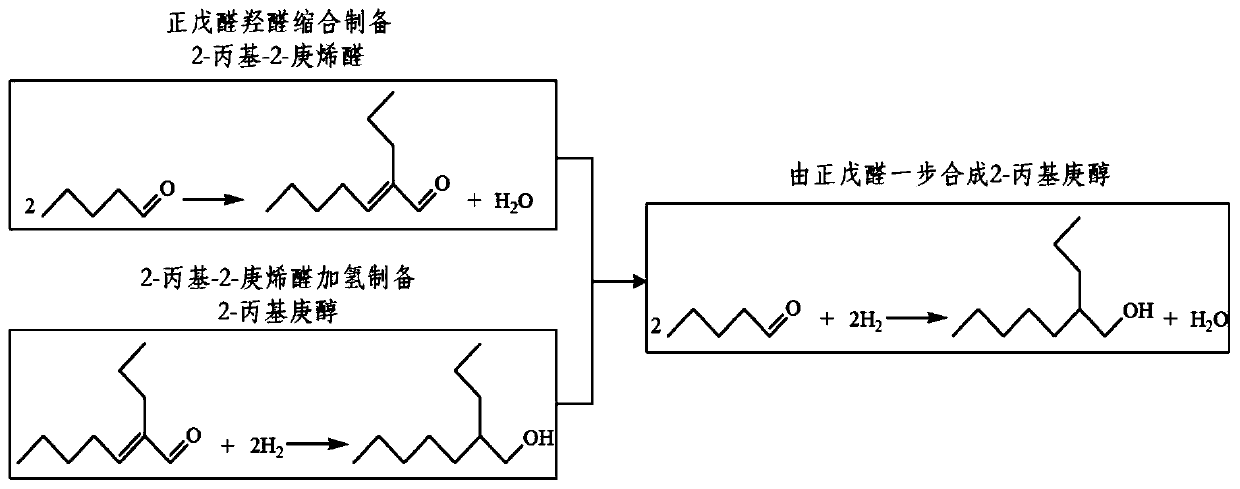
Synthesis method of 2-propyl-2-heptenal
ActiveCN103145536BImprove conversion rateLess side effectsOrganic compound preparationCarbonyl compound preparationPtru catalystReaction temperature
The invention discloses a synthesis method of 2-propyl-2-heptenal, which is implemented through carrying out dimerization reaction by using a solid base catalyst at a specific mass ratio of a raw material n-valeraldehyde and the catalyst under the conditions of specific reaction temperature, reaction time and reaction pressure, wherein the reaction conditions are relatively mild, the requirements on equipment are low, the occurrence of side reaction can be reduced, and a target product 2-propyl-2-heptenal is produced, the conversion rate is as high as 98%, the product yield is over 95%, and the purity is above 98%. The catalyst adopted by the invention is the solid base catalyst; compared with a liquid catalyst, the post-treatment process of the solid base catalyst is simple, and reaction liquid is neutralized without an acid solution, thereby avoiding the environment pollution caused by spent acid wastewater; and the solid base catalyst can be repeatedly used after being subjected to regeneration treatment, thereby reducing the production cost.
Owner:CHAMBROAD CHEM IND RES INST CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com