Process method for one-step synthesis of long-chain alcohol by catalyzing aldehydes with solid catalyst
A technology for catalyzing aldehydes and solid catalysts with catalysts, applied in the field of green chemistry, can solve the problems of catalysts that cannot be reused, large waste water discharge, environmental pollution, etc. short process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] Taking the preparation of Ni-Ce-HY catalyst as an example, the preparation process
[0022] The solid acid HY was calcined in a muffle furnace at 500°C for 4h for later use. First, weigh the metal component precursor Ni(NO 3 ) 2 ·6H 2 O2.664g and auxiliary precursor Ce(NO 3 ) 3 ·6H 2 O0.543g was made into 15mL aqueous solution, and the prepared salt solution was immersed in 10g of heat-treated solid acid HY; after aging for 24h, it was dried at 110°C for 10h, then roasted in a muffle furnace at 450°C for 4h, and finally, 5wt.%Ni-Ce-HY catalyst can be obtained by reducing at 450℃ for 4h in the hydrogen atmosphere of the reduction furnace, in which the mass fraction of Ni is 5%, and the mass fraction of additives is 2%.
Embodiment 1
[0024] Add 30g of n-valeraldehyde and a Ni-Ce-HY catalyst equivalent to 10% of the mass of n-valeraldehyde into a 100mL autoclave, first use N 2 Displacing the air, followed by H 2 Replacement, at a reaction temperature of 160°C, filled with H 2 Maintain the pressure at 2.0MPa, and magnetically stir for 6h. After the reaction, the product liquid was analyzed by gas chromatography, and the conversion rate of n-valeraldehyde was 89.2%, and the yield of decanol was 75.0%. At the same time, a small amount of n-valeraldehyde was directly hydrogenated to generate n-pentanol, and the yield of n-pentanol was 11.8%.
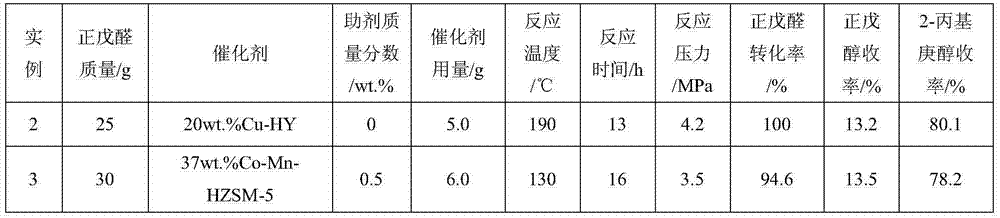
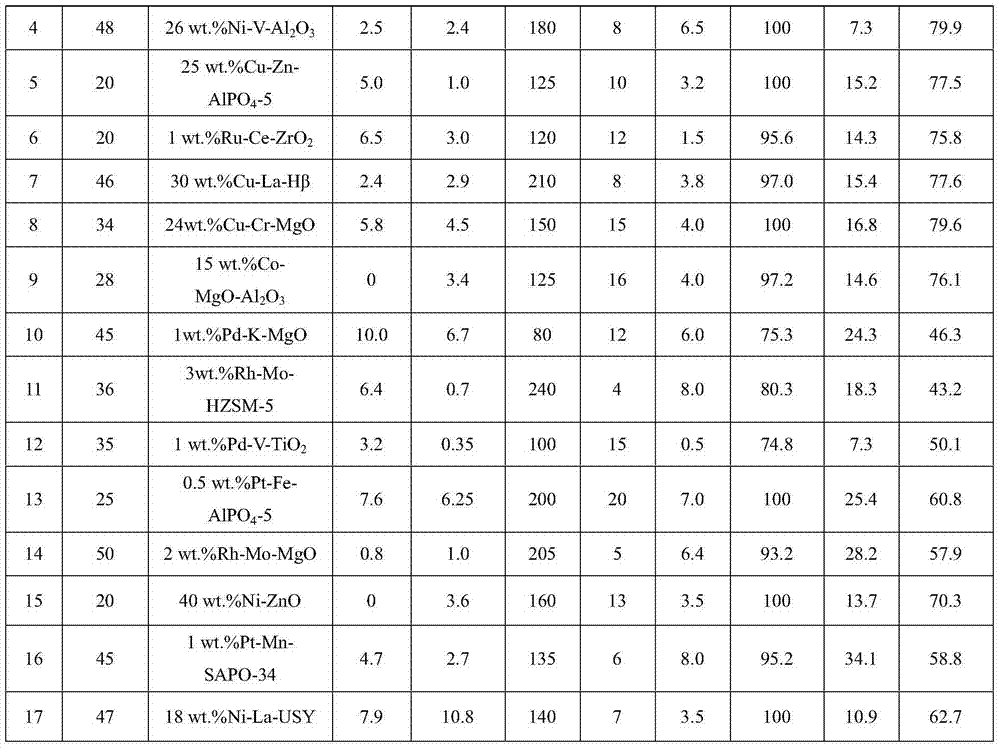
[0025] Examples 2-17 According to the operation steps of Example 1, the reaction conditions and results are shown in the summary table.
[0026]
[0027]
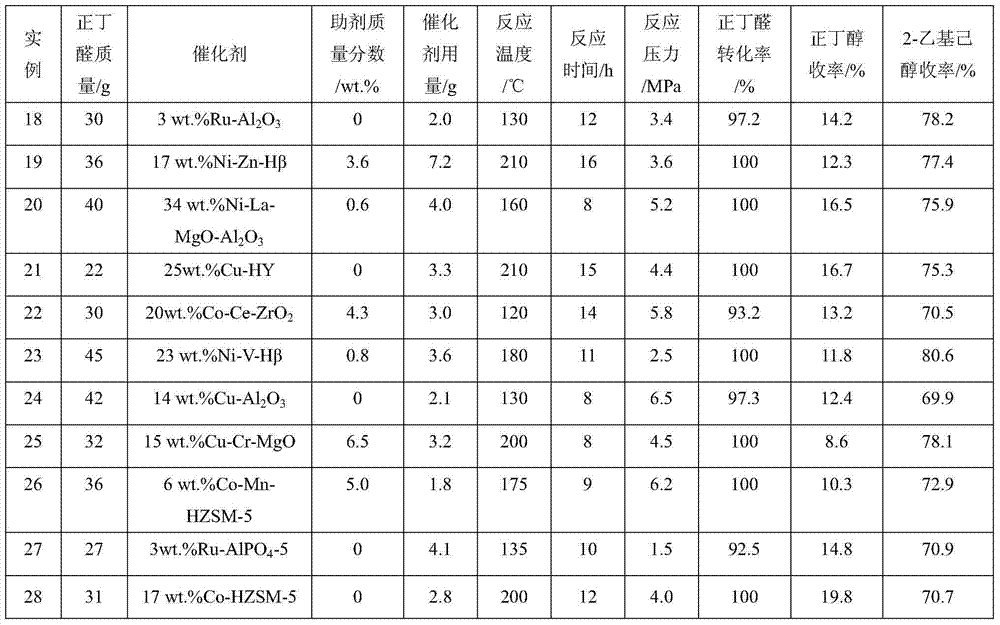
[0028] Examples 18-38 According to the operation steps of Example 1, n-butyraldehyde was selected as the raw material aldehyde, and the reaction conditions and results are shown in the summary table.
[0029]...
Embodiment 39
[0032] In a 100mL autoclave, put 32g n-butyraldehyde, and then add 15wt.%Cu-Cr-MgO catalyst with 10% n-butyraldehyde mass, first use N 2 Purge three times with H 2 Purge three times. flushed with H at 200 °C 2 Maintain the reaction pressure at 4.5MPa, and magnetically stir for 8h. After the reaction was finished, it was filtered under reduced pressure, and the filtrate was analyzed by gas chromatography. The conversion rate of n-butyraldehyde was 100%, and the yield of 2-ethylhexanol was 78.1%. The reacted catalyst was washed three times with absolute ethanol, and dried at 110° C. for 8 h. Roasted in a muffle furnace at 500°C for 4h, and then heated with H 2 Reduction at 200°C for 4h. Then under the same reaction conditions, the repeated use effect of the catalyst was investigated, and the results are shown in the table below. With the increase of the number of reactions, the activity of the catalyst decreased slightly, and the yield of 2-ethylhexanol decreased by 5.4% a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com