Method for preparing aldehyde through linear chain olefin hydroformylation
A technology of olefin hydroformyl and straight chain, applied in the direction of carbon monoxide reaction preparation, chemical instruments and methods, organic compound/hydride/coordination complex catalyst, etc., can solve the problem of expensive phosphine ligands, butene-2 hydrogen Problems such as low catalytic activity of formylation and high catalytic reaction pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
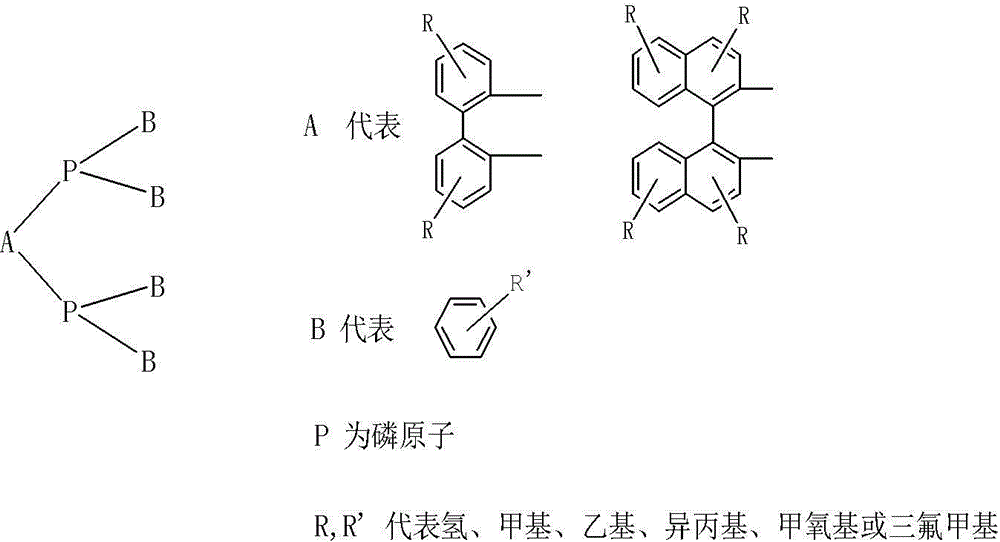
Image
Examples
example 1
[0021] In a 1 liter autoclave, add 700 ml of n-butyraldehyde, rhodium complex HRh(CO)(TPP) 31.50 g, 8.9 g of the bisphosphine ligand 2,2-dimethylene-1,1′-biphenyl-diphenylphosphine (BISBI), and after replacing it with synthesis gas for 4 times, the temperature of the reactor was raised to 90 °C, continuously feed propylene and syngas, the propylene addition rate is 40 g / h (0.952 mol / h), the synthesis gas addition rate is H 2 1.05mol / h and CO 0.952mol / h, keep the total pressure of the reactor at 2MPa; sampling analysis shows that the conversion rate of propylene is 92.5%, and the selectivity of forming butyraldehyde is 98.4%, of which the n-butyraldehyde content is 98.5%.
example 2
[0023] In a 1 liter autoclave, add 700 ml of n-butyraldehyde, rhodium complex HRh(CO)(TPP) 3 1.50 g, 8.9 g of bisphosphine ligand 2,2-dimethylene-1,1′-biphenyl-diphenylphosphine (BISBI), 5 g of triphenylphosphine, replaced with synthesis gas for 4 times, and then The temperature of the reactor is raised to 90°C, and propylene and synthesis gas are continuously added at a rate of 40 g / h (0.952 mol / h) for propylene and H for synthesis gas. 2 1.05mol / h and CO 0.952mol / h, keep the total pressure of the reactor at 2MPa; sampling analysis shows that the conversion rate of propylene is 90.5%, and the selectivity of forming butyraldehyde is 98.1%, of which the n-butyraldehyde content is 97.6%.
example 3
[0025] In a 1 liter autoclave, add solvent toluene 700ml, rhodium complex HRh(CO)(TPP) 3 1.50 g, 9.1 g of the bisphosphine ligand 2,2-dimethylene-1,1′-biphenyl-diphenylphosphine (BISBI), and after replacing it with synthesis gas for 4 times, the temperature of the reactor was raised to 100 °C, butene-1 and synthesis gas are continuously added, butene-1 is added at a rate of 30 g / h (0.536 mol / h), and synthesis gas is added at a rate of H 2 is 0.590mol / h and CO is 0.536mol / h, and the total pressure of the reactor is kept at 2MPa; sampling analysis shows that the conversion rate of butene-1 is 80.5%, and the selectivity of generating valeraldehyde is 98.1%, wherein the content of n-valeraldehyde 97.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com