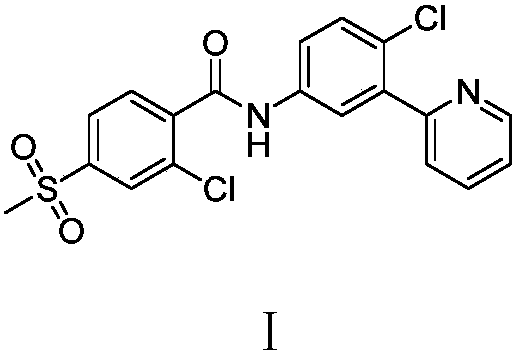
Preparation method of vimodegil
A technology of compound and molar ratio, applied in the field of preparation of vemodaji, can solve the problems of high price of 2-haloacrolein, low product stability, low total yield, etc., and achieve high reaction selectivity, purity and total yield. The effect of high yield, product purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
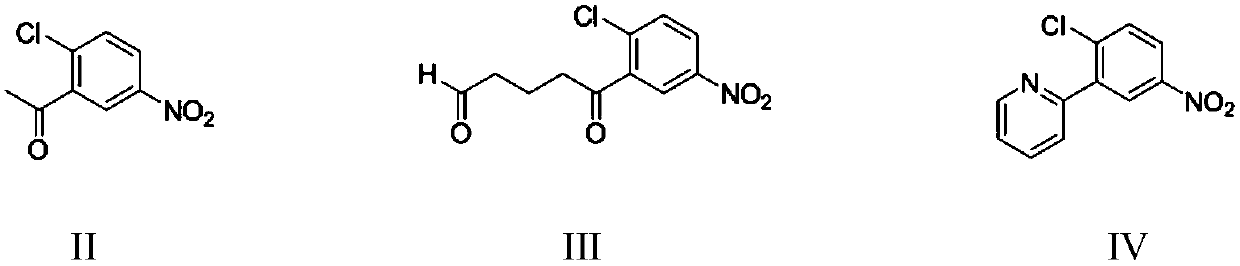
[0073] Embodiment 1: Preparation of 2-(2-chloro-5-nitrophenyl)pyridine (IV)
[0074] Add 200 grams of 1,2-dichloroethane, 20.0 grams (0.1 moles) of 2-chloro-5-nitroacetophenone, 0.5 grams of piperidine, and 6.2 grams (0.11 moles) of acrolein into a 500 mL four-neck flask, React at 30-35°C for 4 hours, lower the reaction solution to 25°C, transfer it to a constant pressure dropping funnel, and drop it into another 500mL four-necked flask containing 85 grams of 10% ammonia water at 20-25°C. The addition was completed in 1 hour, and then the reaction was stirred at 25-30°C for 3 hours. Then add 17.0 grams (0.15 moles) of 30wt% hydrogen peroxide, stir and react at 40-45°C for 3 hours, cool to 20-25°C, separate layers, and extract the water layer twice with 1,2-dichloroethane, 30 grams each time , the combined organic phases were washed once with 20 gram of 5% sodium sulfite aqueous solution, the organic phase was distilled to reclaim the solvent, and dried to obtain 21.5 gram of ...
Embodiment 2
[0075] Embodiment 2: Preparation of 2-(2-chloro-5-nitrophenyl)pyridine (IV)
[0076] Add 200 grams of tetrahydrofuran, 20.0 grams (0.1 moles) of 2-chloro-5-nitroacetophenone, 0.5 grams of DBU, 6.2 grams (0.11 moles) of acrolein into a 500 mL four-neck flask, and react at 25-30 ° C for 4 hours. Transfer the reaction solution to a constant pressure dropping funnel, and drop it into another 500mL four-neck flask containing 85 grams of 10% ammonia water at 20-25°C, and add it in 1 hour, then stir the reaction at 25-30°C 3 Hour. Then add 29.3 grams (0.13 moles) of 40% tert-butanol peroxide, 30-35 DEG C stirred and reacted for 4 hours, cooled to 20-25 DEG C, separated layers, and the aqueous layer was extracted 3 times with dichloromethane, each 50 grams, Combine the organic phases, wash once with 20 grams of 5% sodium sulfite aqueous solution, distill the organic phase to reclaim the solvent, dry to obtain 22.3 grams of 2-(2-chloro-5-nitrophenyl)pyridine, the liquid phase purity i...
Embodiment 3
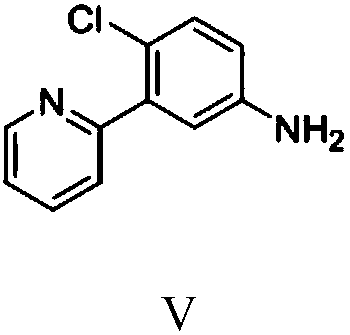
[0077] Embodiment 3: Preparation of 2-(2-chloro-5-aminophenyl)pyridine (Ⅴ)
[0078] 23.5 grams (0.1 moles) of 2-(2-chloro-5-nitrophenyl) pyridine prepared by the method in Example 2, 150 grams of isopropanol, and 0.25 grams of 5% palladium carbon were put into a 500 mL autoclave. After nitrogen replacement 3 times, fill the hydrogen pressure to 0.2-0.3MPa, heat up to 40-50°C, keep the temperature for 3 hours, after the hydrogenation reaction, cool down to room temperature, filter and separate palladium carbon, and recover isopropanol to dryness , to obtain 19.8 g of 2-(2-chloro-5-aminophenyl)pyridine with a liquid phase purity of 99.6% and a yield of 96.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com