Supported metal-acidic ionic liquid catalyst and application thereof
A technology of acidic ionic liquid and catalyst, which is applied in catalytic reaction, physical/chemical process catalyst, organic compound/hydride/coordination complex catalyst, etc. Recycling and other issues, to achieve the effect of easy separation, high catalytic activity and selectivity, and increase the value of industrial applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
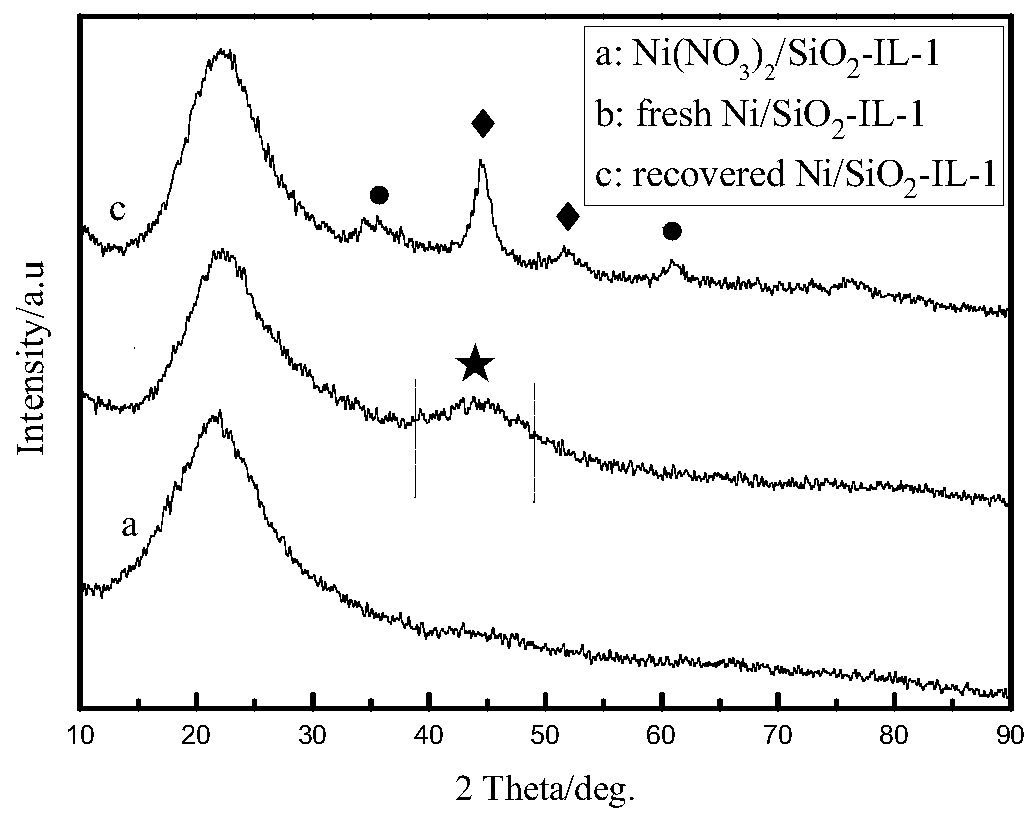
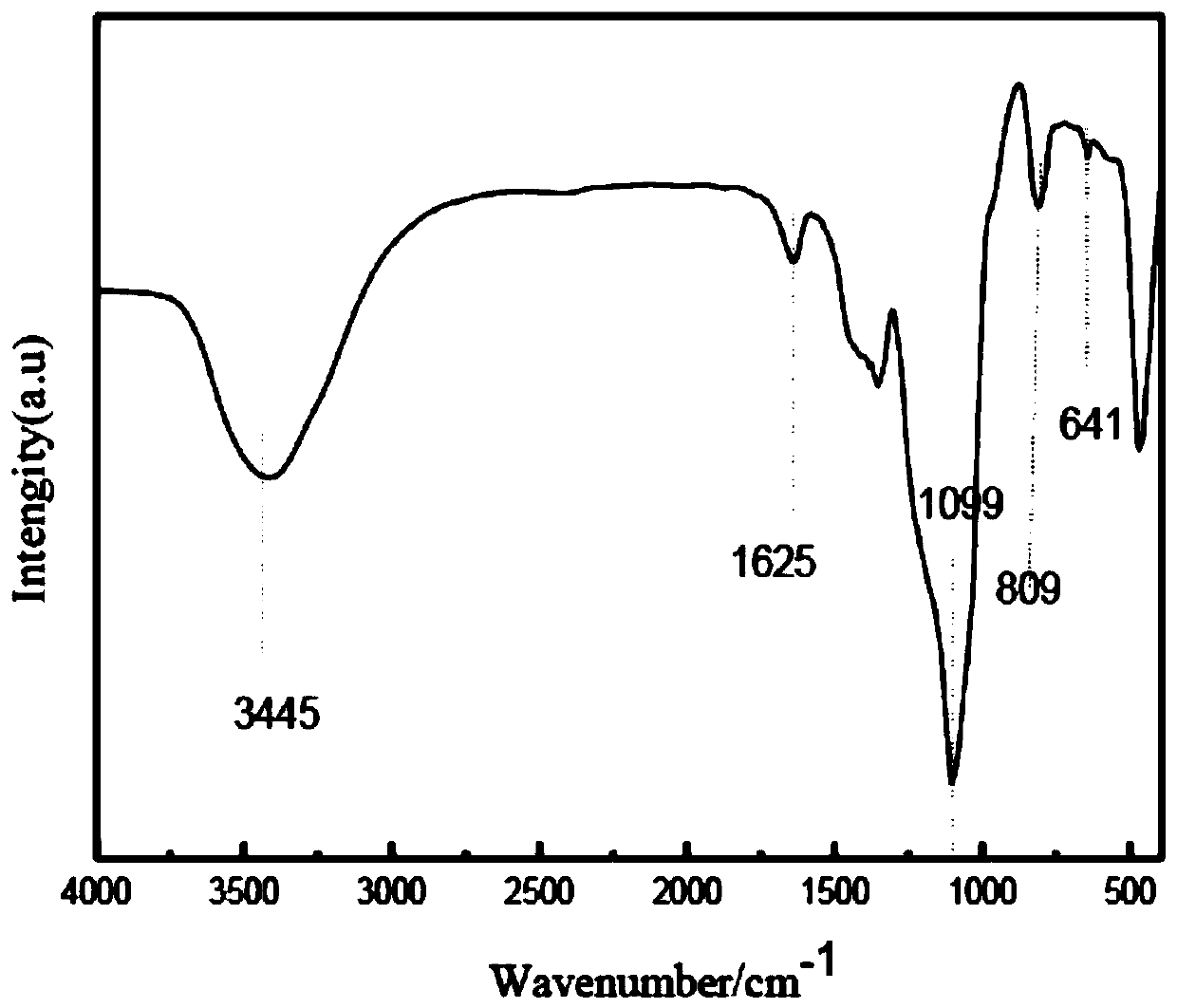
Image
Examples
Embodiment Construction
[0020] In order to better understand the present invention, the present invention will be described in detail below in conjunction with the accompanying drawings.
[0021] 1. Experimental content
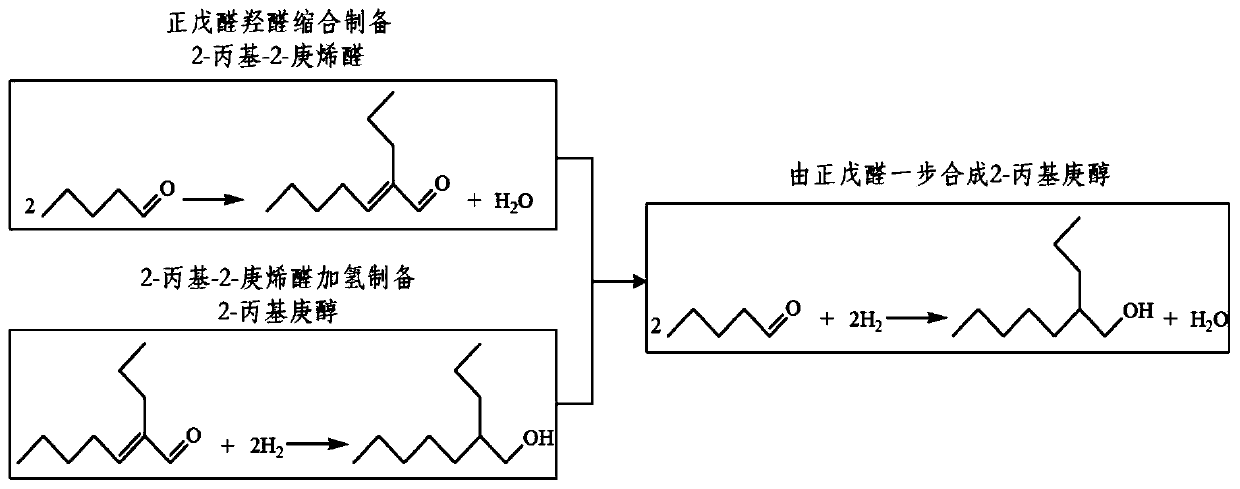
[0022] The internal reaction mechanism of n-valeraldehyde self-condensation to 2-propylheptanol includes two processes: one is the aldol condensation of n-valeraldehyde to obtain 2-propyl-2-heptenal, and the other is 2-propyl-2-heptenal Hydrogenation of alkenals yields 2-propylheptanol ( figure 1 ). The above two reaction mechanisms can be integrated under suitable catalysts and conditions. After regulating the aldol condensation of n-valeraldehyde to generate 2-propyl-2-heptenal, 2-propyl-2-heptenal is then added The hydrogen reaction produces products, and the two mechanisms are promoted synergistically. The rapid consumption of intermediate products can promote the reaction balance to shift to the product direction, which is conducive to improving the efficiency of the reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com