Alcohol dehydrogenase mutant and application thereof to synthesis of chiral biaryl alcohols
An alcohol dehydrogenase and mutant technology, which can be applied in the field of bioengineering, can solve the problems of low stereoselectivity of alcohol dehydrogenase, and achieve the effect of good industrial application prospect and high industrial application value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the activity determination method of alcohol dehydrogenase:
[0047] The total reaction system is 200 μL, including: 1.0mM NADPH, 1.0mM substrate CPMK, sodium phosphate buffer (PBS, 100mM, pH 7.0), mix well, keep warm at 30°C for 2min, add an appropriate amount of enzyme solution, and detect light at 340nm Absorption changes.
[0048] Enzyme activity was calculated using the following formula:
[0049] Enzyme activity (U) = EW × V × 10 3 / (6220×l)
[0050] In the formula, EW is the change of absorbance at 340nm within 1 minute; V is the volume of the reaction solution, the unit is mL; 6220 is the molar extinction coefficient of NADPH, the unit is L / (mol cm); 1 is the optical path distance, the unit is for cm. One activity unit (U) corresponds to the amount of enzyme required to catalyze the oxidation of 1 μmol NADPH per minute under the above conditions.
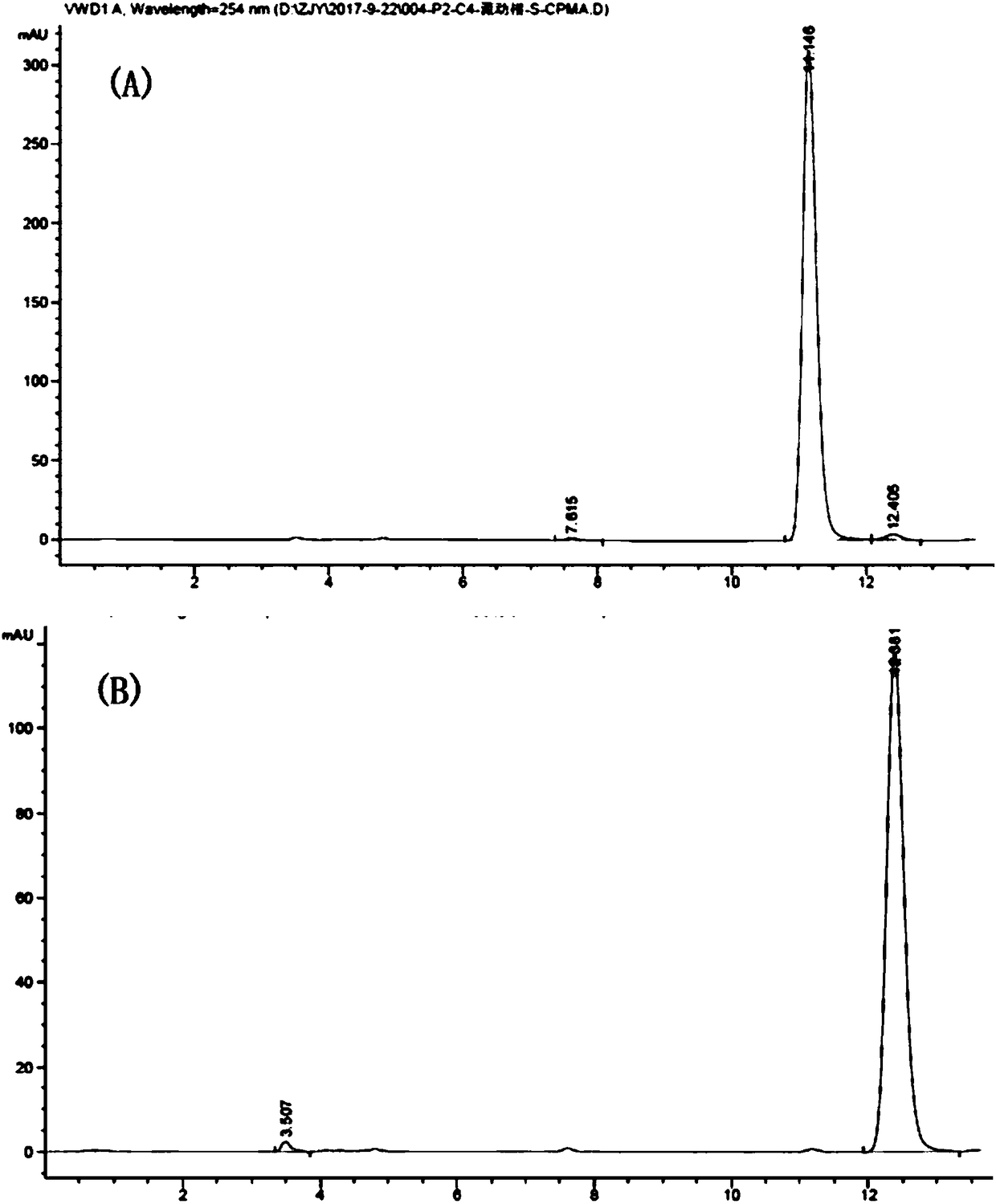
[0051] Determination method of optical purity ee:
[0052] As: the molar concentration of (S)...
Embodiment 2
[0053] Example 2: Construction of Alcohol Dehydrogenase Mutant Gene and Recombinant Expression Transformant
[0054] The whole plasmid PCR method was used to perform site-directed mutations on the amino acid residues at positions 136, 161, 196, 214, 215, and 237 to construct iterative combination mutants. The primers are designed as follows (both are described in the 5'-3' direction, and the underline represents the mutation site:
[0055] M1 (using the pET28a-KpADH recombinant plasmid as a template)
[0056] E214G-F:AAGAAACTAAAT GGTACT TGT
[0057] E214G-R:AAT TTCACAAGT ACC ATT TAG
[0058] M2 (using the pET28a-KpADH recombinant plasmid as a template)
[0059] E214V-F:AAGAAACTAAAT GTT ACT TGT
[0060] E214V-R: AATTTCACAAGT AAC ATT TAG
[0061] M3 (using the M1 recombinant plasmid as a template)
[0062] S237C-F:AAGACTCACTTC TGT CAATTC
[0063] S237C-R:ATCAATGAATTG ACA GAAG TG
[0064] M4 (using the M3 recombinant plasmid as a template)
[0065] Q136N-F: ACC...
Embodiment 3
[0085] Example 3: Expression and purification of alcohol dehydrogenase and mutants thereof
[0086] The recombinant Escherichia coli carrying the stereoselective improvement mutant was inoculated into the LB medium containing kanamycin sulfate (50 μg / mL) by 2% of the transfer amount, 37 ° C, 200 rpm shaker culture, the absorbance of the culture solution OD 600 When it reaches 0.8, add 0.2mM isopropyl-β-D-six-generation galactofuranoside (IPTG) for induction, the induction temperature is 25°C, after induction for 8h, centrifuge at 8000rpm for 10min to obtain highly expressed recombinant alcohol dehydrogenase mutation The collected bacterial cells were suspended in potassium phosphate buffer (100 mM, pH 6.0) and ultrasonically disrupted.
[0087] The column used for purification is HisTrap FF crude, a nickel affinity column, which is accomplished by affinity chromatography using the histidine tag on the recombinant protein. First use solution A to equilibrate the nickel column...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com