Method for preparing fatty aldehyde by means of a hydroformylation reaction
A technology of hydrogenation reaction and hydroformylation, which is applied in the field of preparation of fatty aldehydes by hydroformylation reaction, can solve the problems of increasing the complexity of the process, high price, easy oxidation and hydrolysis, etc., to maintain catalyst activity and selectivity, simplify The production process, the effect of not being easily oxidized and hydrolyzed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
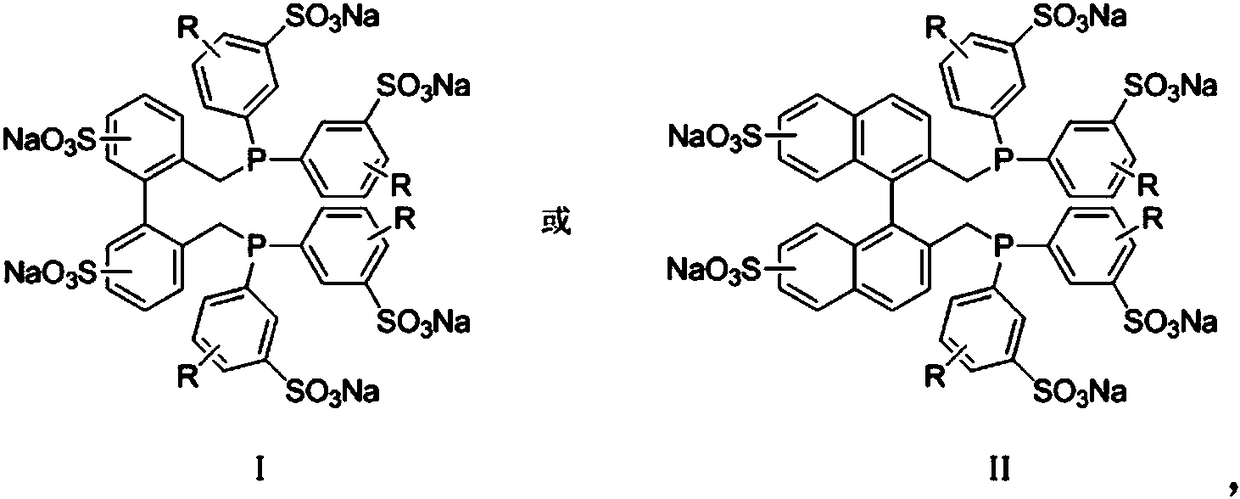
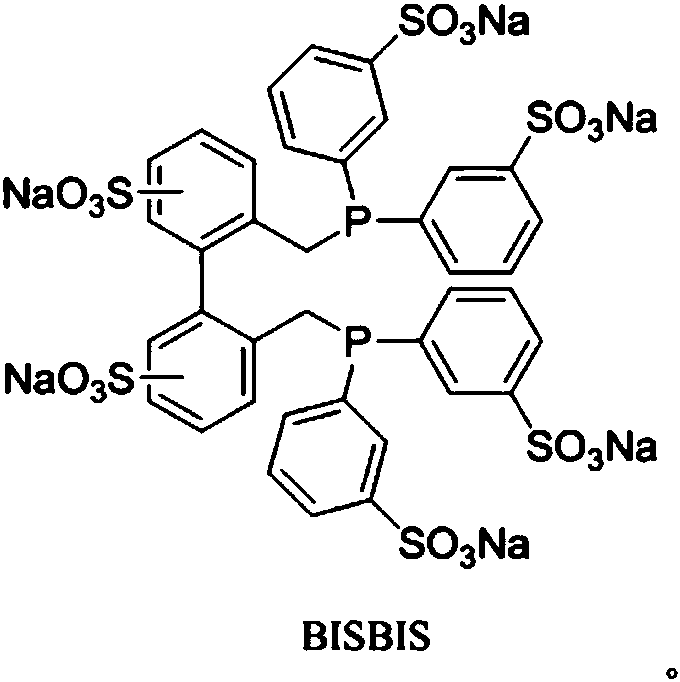
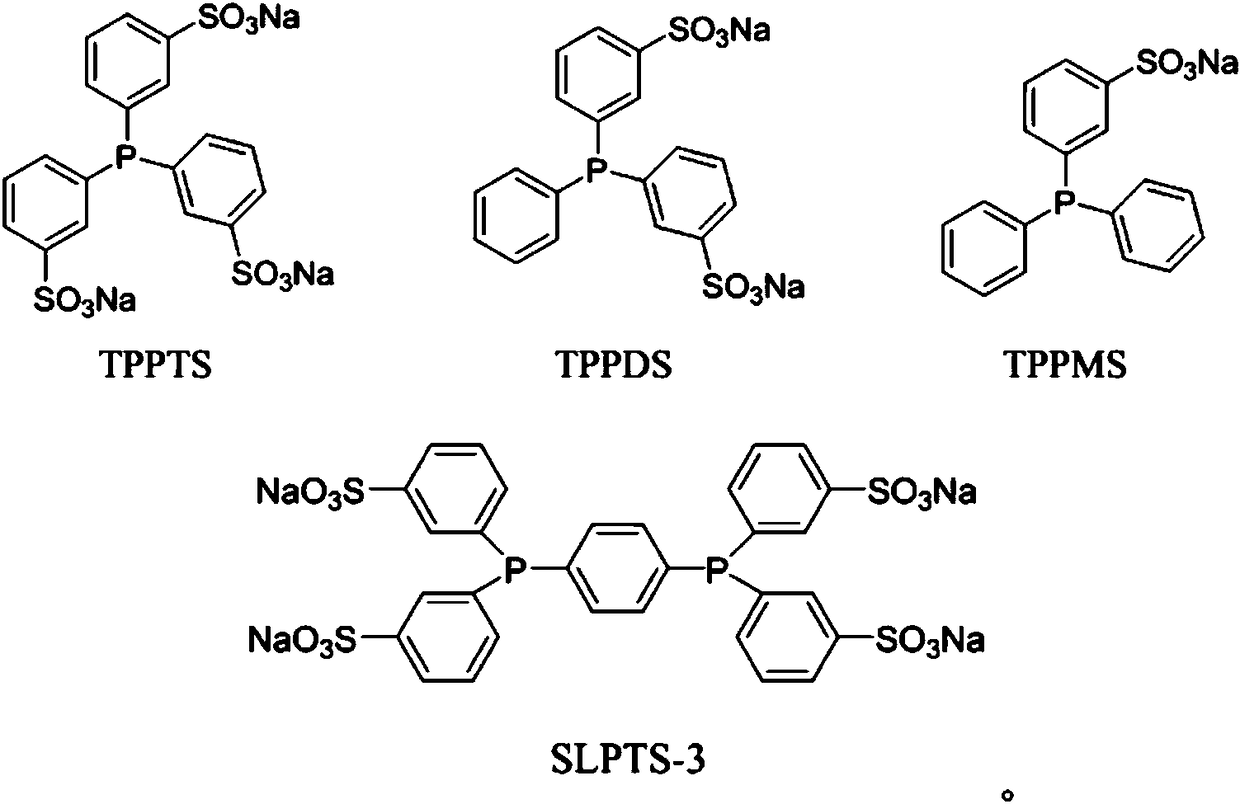
[0037]Add 500 mL of water, 100 mL of n-valeraldehyde and 100 mL of polyethoxylate HO-(-CH 2 CH 2 O) 4 CH 2 CH 2 OH, followed by rhodium complex HRh(CO)(TPPTS) 3 , BISBIS and TPPTS, so that the rhodium concentration in the aqueous solution reaches 300ppm, the molar ratio of BISBIS / Rh is 15:1, and the molar ratio of BISBIS / TPPTS is 6:1. After stirring and mixing, introduce nitrogen to replace the air in the kettle for 3 times, and the reactor After being evacuated, the ratio of 1-butene and 2-butene is 21%: 79% mixed butene 70g is added in the reactor by the feed pump, and then H 2 : CO = 1.1:1 molar ratio synthesis gas is added in the kettle from a high-pressure cylinder, at a reaction temperature of 120° C., a stirring speed of 400 rpm, and a constant pressure of 2.5 MPa in the kettle to react for 3 hours, and then pass water to cool the kettle. When the temperature reaches room temperature, the gas in the reactor is vented, and all the reaction liquid is taken out. It ca...
Embodiment 2
[0039] Carry out mixed butene hydroformylation reaction by the method for embodiment 1, its difference is that the rhodium complex that adopts is RhCl (TPPTS) 3 , organic additives are 50mL anisole and 150mL polyethoxy ether HO-(-CH 2 CH 2 O) 8 CH 2 CH 2 OH, the concentration of rhodium in the aqueous solution is 200ppm, the molar ratio of BISBIS / Rh is 10:1, the molar ratio of BISBIS / TPPTS is 8:1, the reaction is 3h, the conversion rate of mixed butene is 75%, and the selectivity of forming valeraldehyde is 93% , the ratio of n-valeraldehyde to isovaleraldehyde is 22:1, the remaining products are n-amyl alcohol 6.1%, and condensates 0.9%.
Embodiment 3
[0041] Carry out mixed butene hydroformylation reaction by the method for embodiment 1, its difference is that the rhodium complex that adopts is HRh (CO) 2 (BISBIS), the rhodium concentration in the aqueous solution is 400ppm, and the organic auxiliary agent is each 100mL of toluene and n-valeraldehyde. After 4 hours of reaction, the conversion of mixed butenes was 83%, the selectivity of forming valeraldehyde was 93%, the ratio of n-valeraldehyde to isovaleraldehyde was 25:1, and the remaining products were 6.3% of n-pentanol and condensate of 0.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com