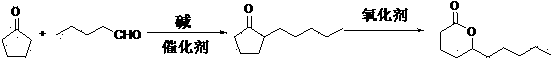Preparation method for delta-decalactone
A technology of decanolactone and butyl-position, which is applied in the field of preparation of butyl-decalactone, can solve the problems of low total yield, long reaction route, and difficulty in industrial production, and achieve fewer steps, lower energy consumption, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] (1) Synthesis of 2-pentylcyclopentanone
[0020] 890g 1.2% sodium hydroxide solution, 4.5gPd / C are added in the 5L reactor, N 2 Replace three times, raise the temperature to 80°C, pass in hydrogen to keep the pressure at 3.0Mpa, add dropwise a mixture of 540g (6.42mol) cyclopentanone and 450g (5.22mol) n-valeraldehyde, dropwise add in 4-5h, continue to keep warm after dropwise Pressure-holding reaction for 0.5h, the reaction is over, release the pressure, N 2 Replaced three times, filtered after cooling down to 60-70°C, recovered the catalyst and used it mechanically, separated the layers, and distilled the oil layer under reduced pressure to obtain 696g of 2-pentylcyclopentanone, the content of which was analyzed by GC was 95%.
[0021] (2) Synthesis of butyl-decalactone
[0022] Add 300g of toluene to the three-necked flask as a solvent, then add 696g of the above-mentioned 2-pentylcyclopentanone, and slowly add 1630g of 20% peroxyacetic acid dropwise under stirring...
Embodiment 2
[0024] (1) Synthesis of 2-pentylcyclopentanone
[0025] 1633g 0.5% potassium hydroxide solution and 40.8g Raney nickel were added to the 5L reactor, N 2 Substitute three times, raise the temperature to 100°C, pass in hydrogen to keep the pressure at 2.0Mpa, add dropwise a mixture of 540g (6.42mol) cyclopentanone and 276.5g (3.21mol) n-valeraldehyde, dropwise add in 4-5h, continue to Heat preservation and pressure reaction for 3h, the reaction is over, release the pressure, N 2 Replaced three times, filtered after cooling down to 60-70°C, recovered the catalyst and used it mechanically, separated the layers, and distilled the oil layer under reduced pressure to obtain 375.8 g of 2-pentylcyclopentanone, the content of which was analyzed by GC was 95%.
[0026] (2) Synthesis of butyl-decalactone
[0027] Add 751.6g of xylene as a solvent to the three-necked flask, then add 375.8g (2.44mol) of the above-mentioned 2-pentylcyclopentanone crude product, and slowly add 179.4g (2.68m...
Embodiment 3
[0029] (1) Synthesis of 2-pentylcyclopentanone
[0030] Add 334.8g 2.0% lithium hydroxide solution, 0.67gPd / C into the 2L reactor, N 2 Substitute three times, raise the temperature to 50°C, pass in hydrogen to keep the pressure at 5.0Mpa, add dropwise a mixture of 219.6g (2.61mol) cyclopentanone and 450g (5.22mol) n-pentanal, dropwise add in 4-5h, continue to Heat preservation and pressure reaction for 15min, the reaction is over, release the pressure, N 2 Replaced three times, filtered after cooling down to 60-70°C, recovered the catalyst and used it mechanically, separated the layers, and distilled the oil layer under reduced pressure to obtain 301.9 g of 2-pentylcyclopentanone, the content of which was analyzed by GC was 95%.
[0031] (2) Synthesis of butyl-decalactone
[0032] Add 15.1g of xylene as a solvent into the three-necked flask, then add 301.9g (1.96mol) of the above-mentioned 2-pentylcyclopentanone, and slowly add 406.1g (2.94mol) of peroxybenzoic acid under st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com