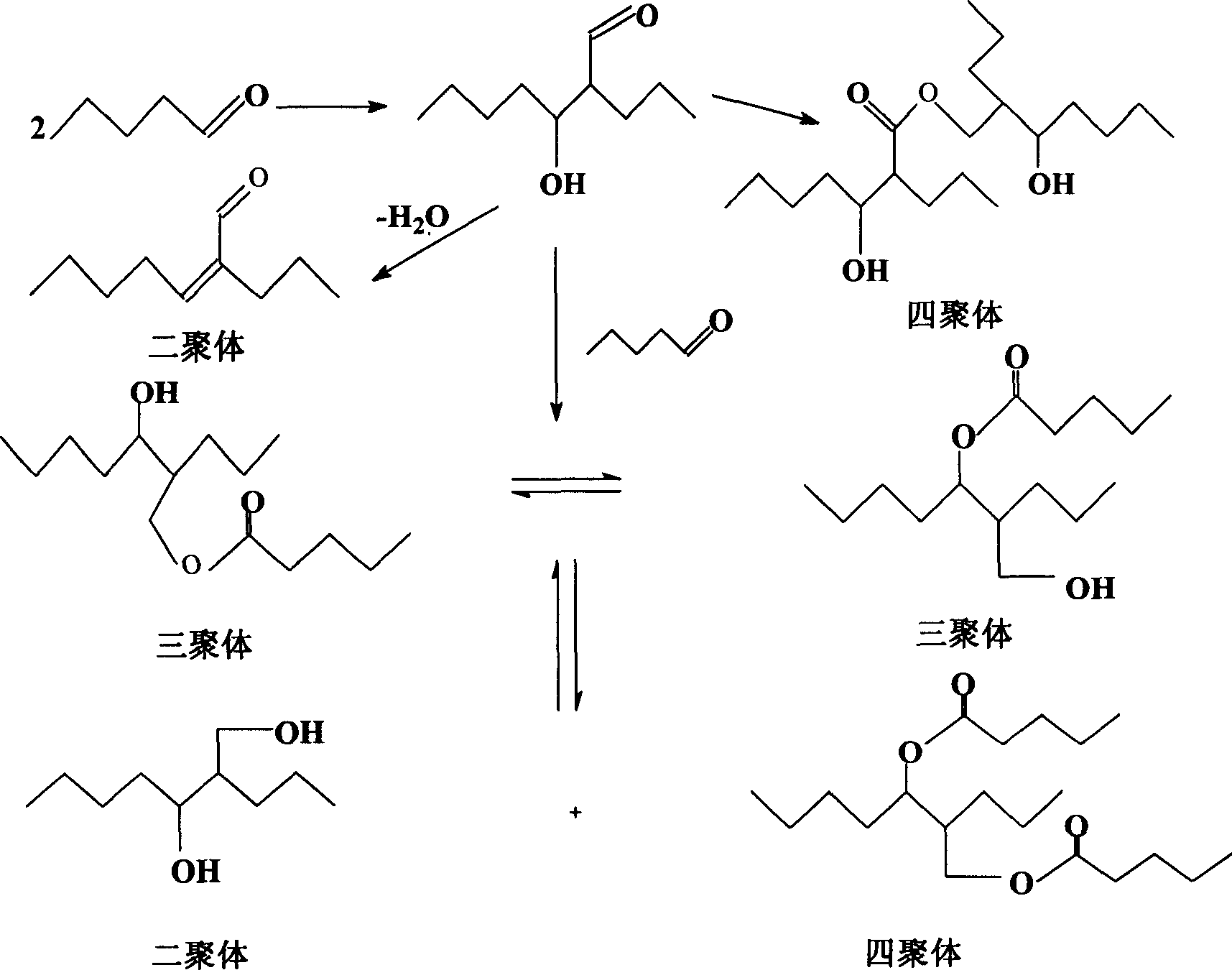
Process of continuously preparing n-pentanal
A process, n-valeraldehyde technology, applied in the field of continuous preparation of n-valeraldehyde by butene hydroformylation reaction, can solve the problem of increasing high-boiling point high polymer, increasing polycondensation of olefin hydroformylated aldehyde products, reducing catalyst activity, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
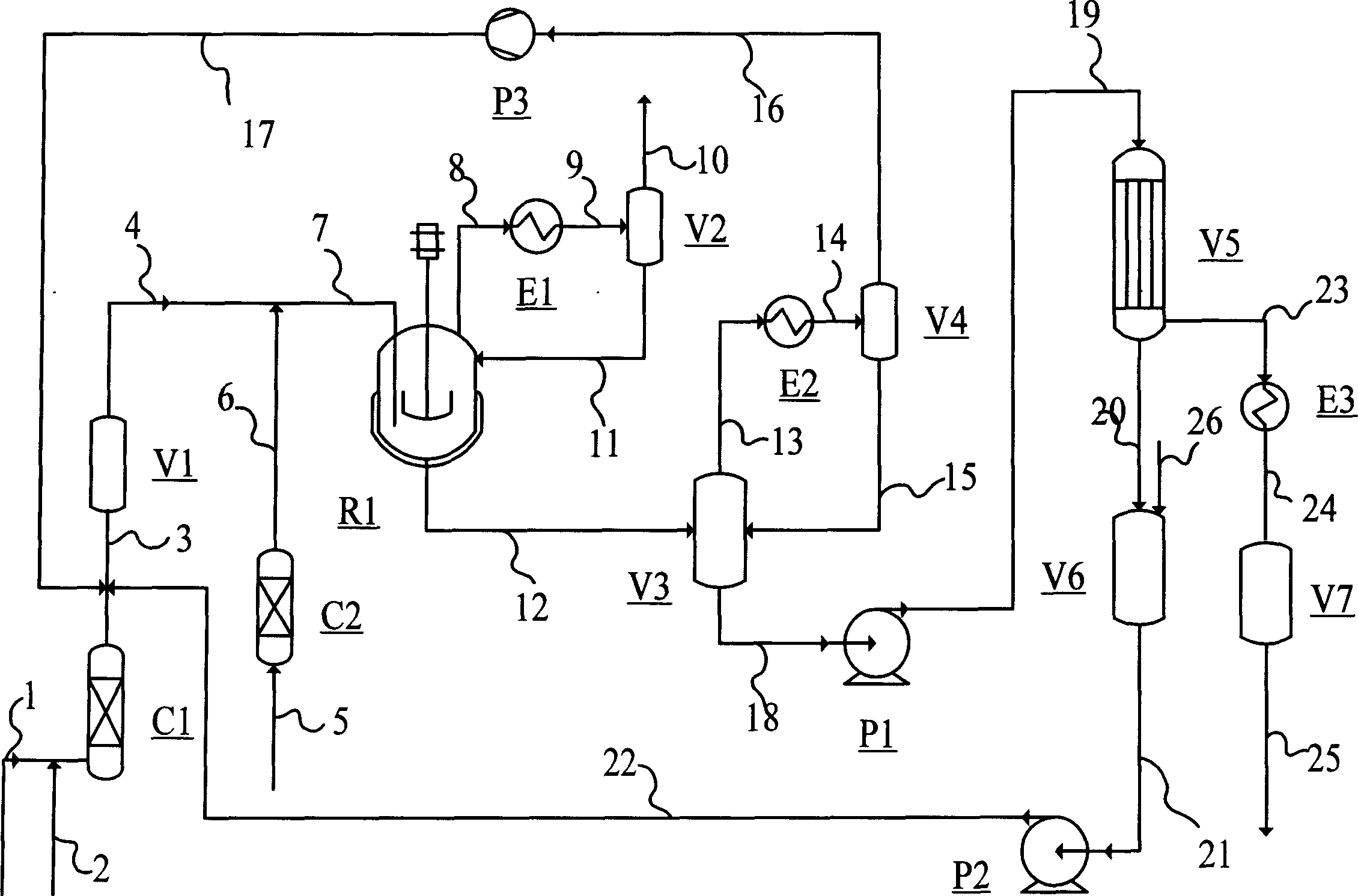
[0056] In the reaction process shown in the accompanying drawing, the volume of the reactor is 1 liter, equipped with a speed-regulating double-layer electromagnetic stirrer, and heated by an oil bath jacket. The catalyst activator is electrically heated.
[0057] Add 3.84 g triphenylphosphine rhodium acetylacetonate and 369.8 g triphenylphosphine catalyst solution uniformly dissolved in 3.5 liters of n-valeraldehyde into the flow reaction system. Purge with high-purity nitrogen to replace the system air. Introduce purified H 2 / CO ratio of 1.0 syngas until the oxygen content of the system is below 0.1ppm. Add 1-butene purity 95.2wt% and isobutene 0.5wt% olefin raw material to react. The operating conditions of reactor R1 are controlled at 80-90°C and 1.9-2.1MPa. The reaction liquid is separated into the liquid phase at 60°C and 0.4-0.8MPa in the gas-liquid reactor V3, and sent to the thin-film evaporator V5 by the pump P1, and the crude product valeraldehyde is separated a...
Embodiment 2
[0060] Butene hydroformylation operation experimental method identical with embodiment 1. Add 1-butene with 80.8wt% purity of 1-butene and 1.5wt% isobutene as olefin raw material, and continue the hydroformylation reaction for 1020 hours. The results are listed in Table 1.
Embodiment 3
[0062] Butene hydroformylation operation experimental method identical with embodiment 1. Butene raw material composition is 1-butene 55.4wt%, cis / trans-2-butene 22.6wt%, butane 19.5wt%, isobutene 2.5wt%, continuous hydroformylation reaction for 50 hours, the results are listed in Table 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com