Preparation method of antiepileptic drug intermediate
A technology of antiepileptic drugs and intermediates, applied in the field of medicine, can solve the problems of difficult Knoevenage condensation and precipitation, and achieve the effects of low cost, mild reaction conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
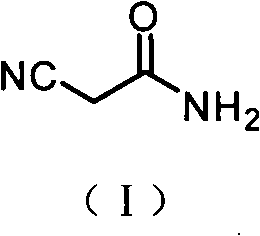
[0022] 1) preparation of cyanoacetamide:
[0023]
[0024] Ethyl cyanoacetate (100.0 g, 0.88 mol) and absolute ethanol (100 mL, 1.72 mol) were placed in a reaction flask, and the system was cooled to 5° C. with an ice bath. Start to feed ammonia gas, remove the ice bath, slowly increase the reaction temperature with the introduction of ammonia gas, control the speed of feeding ammonia gas so that the ammonia gas does not overflow, reach the temperature of 32 ° C, and continue the water bath insulation reaction for 5 hours. Cool, filter with suction, rinse with a small amount of cooled absolute ethanol several times, and dry the product to obtain 71.6g of white solid, yield: 96.4%, m.p.119-120°C.
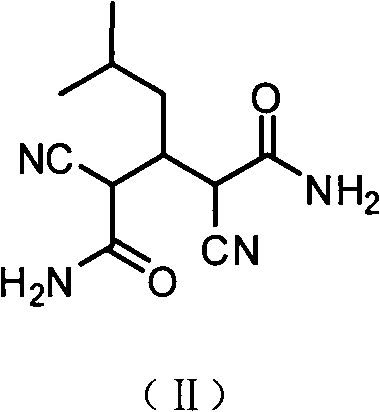
[0025] 2) Preparation of 2,4-dicyano-3-isobutylglutaramide:
[0026]
[0027] Put the above-mentioned cyanoacetamide (100.0g, 1.2mol) and 650mL of water into a reaction flask, heat to dissolve, cool in an ice bath close to 0°C, add a catalytic amount of triethylamine 34mL and ...
Embodiment 2
[0032] 1) preparation of cyanoacetamide:
[0033] Ethyl cyanoacetate (100.0 g, 0.88 mol) and water (500 mL) were placed in a reaction flask, and the system was cooled to 5° C. with an ice bath. Start dripping ammonia water (100ml, 25%), remove the ice bath, slowly increase the reaction temperature with the addition of ammonia water, control the speed of dripping ammonia water so that the reaction temperature is lower than 32°C, and continue the water bath insulation reaction for 5h. Cool, filter with suction, rinse with a small amount of cooled absolute ethanol several times, and dry the product to obtain 56 g of white solid, yield: 80%, m.p.119-120°C.
[0034] 2) Preparation of 2,4-dicyano-3-isobutylglutaramide:
[0035] Put the above cyanoacetamide (100.0g, 1.2mol) and 650mL of water in a reaction flask, heat to dissolve, cool in an ice bath close to 0°C, add a catalytic amount of diethylamine 34mL and isovaleraldehyde (52.0g, 0.6mol) , the reaction system is exothermic, a...
Embodiment 3
[0039]1) preparation of cyanoacetamide:
[0040] With embodiment one, in step 1).
[0041] 2) Preparation of 2,4-dicyano-3-isobutylglutaramide:
[0042] The above-mentioned cyanoacetamide (100.0g, 1.2mol) and 650mL of water were placed in a reaction flask, heated to dissolve, cooled in an ice bath close to 0°C, and a catalytic amount of ammonia water 34mL and isovaleraldehyde (52.0g, 0.6mol) were added to react The system is exothermic, and the reaction temperature is kept at 3-5°C with an ice bath. During the reaction, TLC is used to monitor (developing agent is ethyl acetate: methanol: petroleum ether = 4: 1: 1), and the reaction is 4h. During the reaction White solids were continuously precipitated from the reaction system. Suction filtration, washing with cold water, drying to obtain 100.0 g of white solid, yield: 71.5%, m.p.153-155°C.
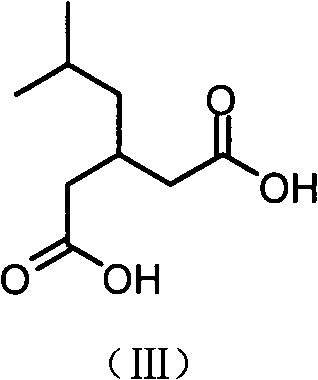
[0043] 3) Preparation of 3-isobutylglutaric acid:
[0044] Take the condensate prepared above (75.0g, 0.39mol), 30mL of concentrated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com