Method for preparing isoamyl aldehyde from 3-methyl-3-butenyl-1 alcohol
A technology of butenyl and isovaleraldehyde, which is applied in the field of photocatalytic reaction for preparing isovaleraldehyde, can solve the problems of difficult control of oxygen supply, high catalyst preparation and cost, short service life, etc., and achieves high conversion rate of effective products, Conducive to the implementation of industrialization, the method is scientific and reasonable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
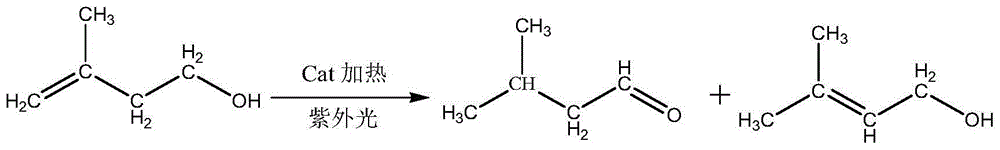
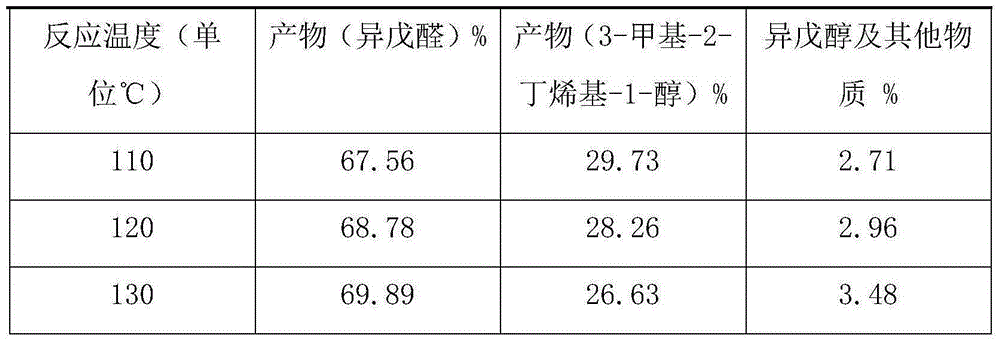
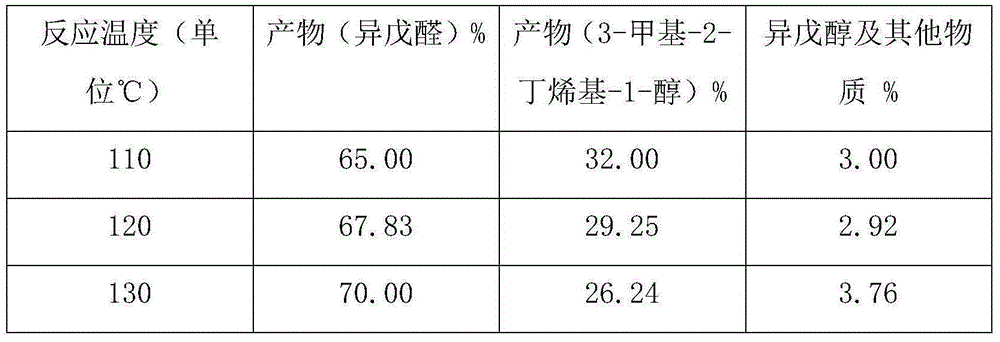
[0012] Example 1: 196g of 3-methyl-3-butenyl-1-alcohol and 4.0g of catalyst were mixed and stirred evenly and added to a 500ml reactor, and placed at a temperature of 140 ℃ The reactor in the oil bath pot, feed nitrogen into the material under normal pressure, replace the air in the reactor to make it anaerobic, and then heat it to the required reaction temperature, 110°C-130°C, in the ultraviolet The reaction starts under light irradiation, and as the reaction progresses, part of the product isovaleraldehyde evaporates through the cooling device on the reactor, and is continuously separated to obtain part of the crude product of isovaleraldehyde; the total reaction time is 3-4h, and the reaction raw materials are all converted The desired products isovaleraldehyde and 3-methyl-2-butenyl-1-ol with a small amount of isopentyl alcohol by-product. After the reaction is finished, the reaction product is rectified by a rectification tower to obtain high-purity isovaleraldehyde and ...
Embodiment 2
[0018] Example 2, 1.572 kg of 3-methyl-3-butenyl-1-ol and 28 g of catalyst were mixed and stirred evenly and added to a 2L reactor, and an ultraviolet lamp was added to a hollow position inside the reactor to pass through the ultraviolet lamp at 254nm Light irradiation, place the reactor in an oil bath at a temperature of 140°C, pass nitrogen into the material under normal pressure, replace the air in the reactor to make it in an anaerobic state, and then heat it to the required reaction temperature of 110°C -130°C, the reaction starts under the irradiation of ultraviolet light. As the reaction progresses, part of the product isovaleraldehyde evaporates through the cooling device on the reactor, and is continuously separated to obtain part of the crude product of isovaleraldehyde; the total reaction time is 3- 4h, the reaction raw materials were all converted to generate the desired product isovaleraldehyde and 3-methyl-2-butenyl-1-ol and a small amount of impurity by-products....
Embodiment 3
[0022] Example 3, 6.895kg of 3-methyl-3-butenyl-1-ol and 105g of catalyst were mixed and stirred evenly and added to a 10L reactor, and an ultraviolet light was added to a hollow position inside the reactor to pass through the ultraviolet light at 254nm Irradiate, place the reactor in the oil bath at a temperature of 140°C, pass nitrogen into the material under normal pressure, replace the air in the reactor to make it in an anaerobic state, and then heat it to the required reaction temperature of 110°C- 130°C, the reaction starts under the irradiation of ultraviolet light. As the reaction progresses, part of the product isovaleraldehyde evaporates through the cooling device on the reactor, and is continuously separated to obtain part of the crude product of isovaleraldehyde; the total reaction time is 3-4h , the reaction raw materials are all converted to generate the desired product isovaleraldehyde and 3-methyl-2-butenyl-1-alcohol and a small amount of impurity by-products. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com