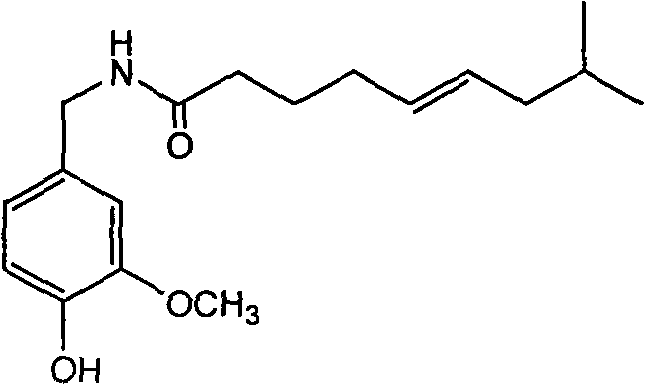
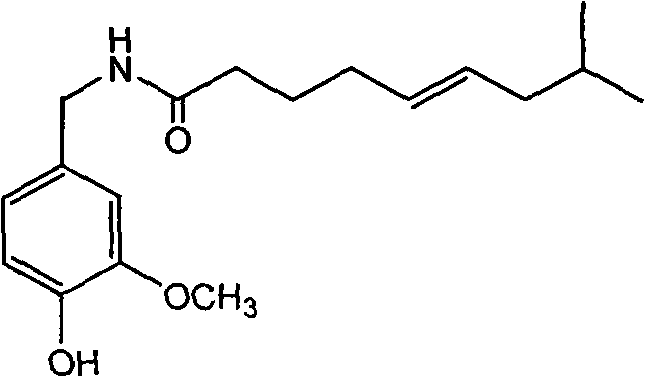
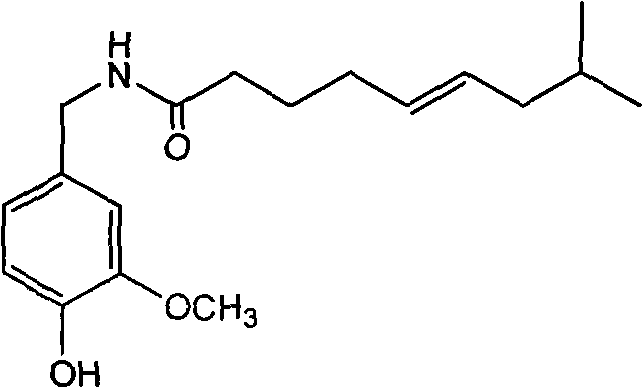
Trans-N-(4- hydroxyl-3-methoxybenzy)-8-methyl-5-nonenamide and preparation method thereof
A technology of methoxybenzyl and nonenamide, applied in the field of capsaicinoids and preparation thereof, can solve problems such as no synthesis report, and achieve the effects of low cost, readily available raw materials, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) 4.0g NaBH 4 Dubbed 50mL aqueous solution, and added a small amount of NaOH. The solution was slowly added dropwise to 100 g of 25% glutaraldehyde solution in an ice-water bath, and reacted for 4 hours after the dropwise addition was completed. Then 1M dilute HCl was added dropwise until pH = 3-4. Add 2M NaOH aqueous solution dropwise to the product to adjust the pH of the product to 8-9; distill off the water under reduced pressure, then add an appropriate amount of acetone and anhydrous Na 2 SO 4 Drying; filtering; the filtrate is distilled under reduced pressure to remove acetone; and then distilled under reduced pressure to collect 1,5-pentanediol product, and the yield can reach 91%.
[0037] Weigh 5.2g of 1,5-pentanediol, 13.1g of 40% hydrobromic acid, and 300mL of n-heptane into a three-necked flask, add an appropriate amount of concentrated sulfuric acid, and react under reflux for 8h. Separate the n-heptane phase with a separatory funnel, and wash the n-...
Embodiment 2
[0049] (1) Mix 8.0g KBH4 into 100mL aqueous solution, and add a small amount of NaOH. The solution was slowly added dropwise to 150 g of 25% glutaraldehyde solution in an ice-water bath, and reacted for 5 hours after the dropwise addition was completed. Then 1M dilute HCl was added dropwise until pH = 3-4. Add 2M NaOH aqueous solution dropwise to the product to adjust the pH of the product to 8-9; distill off the water under reduced pressure, then add an appropriate amount of acetone and anhydrous Na 2 SO 4 Drying; filtering; the filtrate is distilled under reduced pressure to remove acetone; and then distilled under reduced pressure to collect 1,5-pentanediol product with a yield of 88%.
[0050] Weigh 10.4g of 1,5-pentanediol, 30.5g of 40% hydrobromic acid, and 500mL of n-heptane into a three-necked flask, add an appropriate amount of concentrated sulfuric acid, and react under reflux for 8h. Separate the n-heptane phase with a separatory funnel, and wash the n-heptane ph...
Embodiment 3
[0060] (1) 5.8g KBH 4 Dubbed 70mL aqueous solution, and added a small amount of KOH. The solution was slowly added dropwise to 100 g of 25% glutaraldehyde solution in an ice-water bath, and reacted for 5 hours after the dropwise addition was completed. Then 2M dilute HCl was added dropwise to pH=3-4. Add 2M NaOH aqueous solution dropwise to the product to adjust the pH of the product to 8-9; distill off the water under reduced pressure, then add an appropriate amount of acetone and anhydrous Na 2 SO 4 Drying; filtering; the filtrate is distilled under reduced pressure to remove acetone; and then distilled under reduced pressure to collect 1,5-pentanediol product with a yield of 89%.
[0061] Weigh 15.5g of 1,5-pentanediol, 30.5g of 40% hydrobromic acid, and 800mL of n-heptane into a three-necked flask, add an appropriate amount of concentrated sulfuric acid, and react under reflux for 8 hours, while removing the generated water through an oil-water separator . Separate th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com