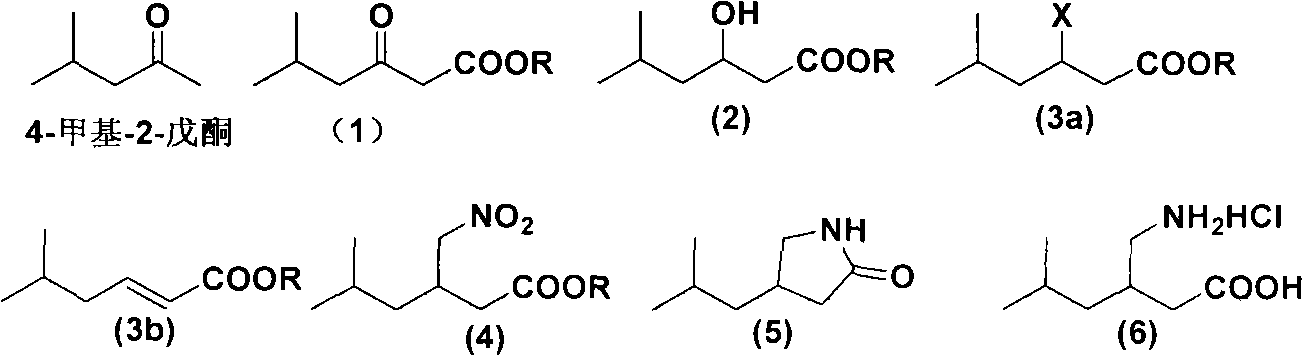
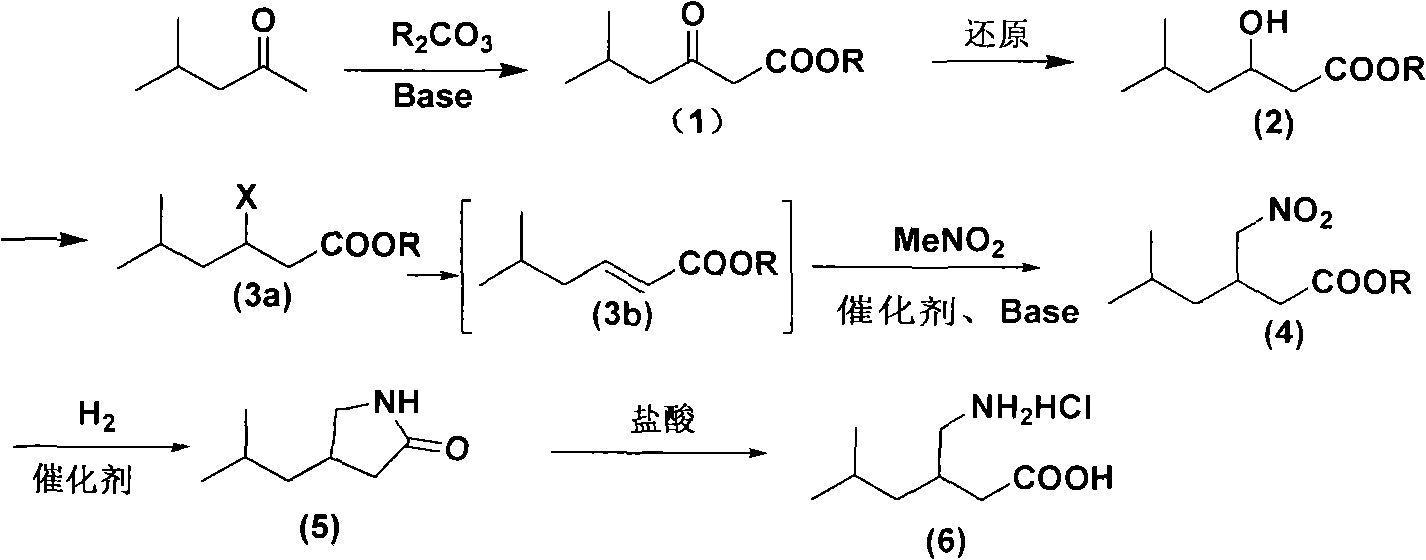
Novel method for preparing pregabalin raceme hydrochloride
A technology of pregabalin and racemate, applied in the field of chemical pharmacy, can solve the problems of cumbersome routes, difficult industrialization, harsh reaction conditions, etc., and achieves the effects of simple operation, low cost and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Add 16g of sodium hydride to a 500ml three-necked flask, then add 70ml of dimethyl carbonate, and stir. Then add 20g of 4-methyl-2-pentanone, heat up to reflux reaction, and the system temperature is about 80°C. After refluxing for about 2 hours, the reaction of 4-methyl-2-pentanone was complete. Stop the reaction, add 200ml of ice water after cooling to room temperature, adjust the pH of the system to about 2-3 with concentrated hydrochloric acid, first extract with 80ml of dichloromethane, and then extract the aqueous phase with 40×2ml of dichloromethane. The combined organic phases were washed with brine and dried over anhydrous sodium sulfate for 2 hours. The desiccant was filtered off, and the solvent was distilled off under reduced pressure to obtain compound (1) as a light brown oil weighing 28.8 g with a yield of about 91%.
Embodiment 2
[0076] Add 16g of sodium hydroxide to a 500ml three-necked flask, then add 70ml of dibutyl carbonate, and stir. Then add 20g of 4-methyl-2-pentanone, heat up to reflux reaction, and the system temperature is about 80°C. After refluxing for about 2 hours, the reaction of 4-methyl-2-pentanone was complete. Stop the reaction, add 200ml of ice water after cooling to room temperature, adjust the pH of the system to about 2-3 with concentrated hydrochloric acid, first extract with 80ml of dichloromethane, and then extract the aqueous phase with 40×2ml of dichloromethane. The combined organic phases were washed with brine and dried over anhydrous sodium sulfate for 2 hours. The desiccant was filtered off, and the solvent was distilled off under reduced pressure to obtain compound (1) as a light brown oil weighing 26.5 g with a yield of about 84%.
Embodiment 3
[0078] Add 21.6g of sodium methoxide to a 500ml three-necked flask, then add 70ml of diethyl carbonate, and stir. Then add 20g of 4-methyl-2-pentanone, heat up to reflux reaction, and the system temperature is about 80°C. After refluxing for about 2 hours, the reaction of 4-methyl-2-pentanone was complete. Stop the reaction, add 200ml of ice water after cooling to room temperature, adjust the pH of the system to about 2-3 with concentrated hydrochloric acid, first extract with 80ml of dichloromethane, and then extract the aqueous phase with 40×2ml of dichloromethane. The combined organic phases were washed with brine and dried over anhydrous sodium sulfate for 2 hours. The desiccant was filtered off, and the solvent was distilled off under reduced pressure to obtain compound (1) as a light brown oil with a yield of about 76%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com