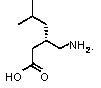
Preparation method of pregabalin
A pregabalin and solvent technology, applied in the field of pregabalin preparation, can solve the problems of unfavorable industrial production, high corrosion of equipment, long reaction time, etc., and achieve the advantages of large-scale industrial production, lower production cost and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
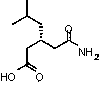
[0022] A preparation method of formula I pregabalin, characterized in that the preparation method comprises the following steps:
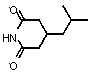
[0023] (a) In 250mL of isopropyl ether, add 45g of 3-(carbamoylmethyl)-5-methylhexanoic acid of formula II, stir to dissolve, and heat up to 45°C; 22.8g of chiral resolving agent (R)-(+)-α-Phenylethylamine was dissolved in 120mL tetrahydrofuran, and slowly added dropwise to the above reaction solution; after dropping, reacted at 45°C for 2 hours; then cooled to room temperature, and gradually solids precipitated. Stir, cool to 5°C to crystallize, filter with suction, place the resulting solid in 300 mL of ether for slurry, and dry to obtain 46 g of 3-(carbamoylmethyl)-5-methylhexanoic acid (R)- (+)-α-Phenylethylamine salt, white solid, yield: 58%; the resulting filtrate was added to 10 times the amount of 5% sodium hydroxide solution, stirred for 30 minutes, the aqueous phase was separated, and adjusted with hydrochloric acid When the pH reached 2...
Embodiment 2
[0029] A preparation method of formula I pregabalin, characterized in that the preparation method comprises the following steps:
[0030] (a) In 700mL of chloroform, add 60g of 3-(carbamoylmethyl)-5-methylhexanoic acid of formula II, stir to dissolve, and heat up to 45°C; 54.8g of chiral resolving agent (R )-(+)-α-Phenylethylamine was dissolved in 200mL tetrahydrofuran, and slowly added dropwise to the above reaction solution; after dropping, reacted at 45°C for 2 hours; then cooled to room temperature, gradually solids were precipitated, stirred, Cool to 5°C for crystallization, filter with suction, place the resulting solid in 400 mL of ether for slurry, and dry to obtain 59.2 g of 3-(carbamoylmethyl)-5-methylhexanoic acid (R)-( +)-α-phenylethylamine salt, white solid, yield: 56%; the obtained filtrate was added to 10 times the amount of 5% sodium hydroxide solution, stirred for 30 minutes, the water phase was separated, and the pH was adjusted with hydrochloric acid To 2-3...
Embodiment 3
[0036] A preparation method of formula I pregabalin, characterized in that the preparation method comprises the following steps:
[0037] (a) In 220mL of toluene, add 20g of 3-(carbamoylmethyl)-5-methylhexanoic acid of formula II, stir to dissolve, and heat up to 45°C; 22.8g of chiral resolving agent (R )-(+)-α-Phenylethylamine was dissolved in 80mL of tetrahydrofuran, and slowly added dropwise to the above reaction solution; after dropping, reacted at 45°C for 2 hours; then cooled to room temperature, gradually solids were precipitated, stirred, Cool to 5°C to crystallize, filter with suction, place the resulting solid in 220 mL of ether for slurry, and dry to obtain 18.3 g of 3-(carbamoylmethyl)-5-methylhexanoic acid (R)-( +)-α-phenylethylamine salt, white solid, yield: 52%; the obtained filtrate was added to 10 times the amount of 5% sodium hydroxide solution, stirred for 30 minutes, the water phase was separated, and the pH was adjusted with hydrochloric acid To 2-3, a so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com