Pregabalin orally disintegrating tablet and dispersible tablet and preparation method thereof
A technology of pregabalin and dispersible tablets, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., which can solve the problem of long-term storage and inconvenient carrying of pregabalin solution by patients and other issues to achieve good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1. Pregabalin orally disintegrating tablets
[0029] The prescription of pregabalin orally disintegrating tablets is shown in Table 1:
[0030] Table 1 Prescription of pregabalin orally disintegrating tablets:
[0031] .
[0032] Preparation:
[0033] 1. Pregabalin, sucralose, mannitol, citric acid, microcrystalline cellulose and crospovidone were mixed in a wet granulator;
[0034] 2. Add appropriate amount of water for granulation;
[0035] 3. The obtained granules are dried and sieved;
[0036] 4. The sieved granules are mixed with other external phases (strawberry essence, mannitol, microcrystalline cellulose, crospovidone, silicon dioxide and sodium stearyl fumarate);
[0037] 5. The final mixture is compressed with a 12mm die to obtain tablets of the required hardness.
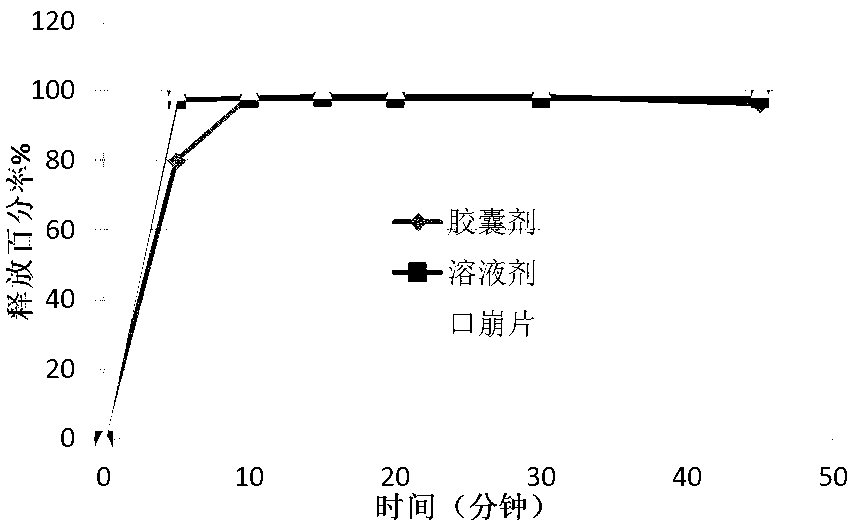
[0038] Disintegration Time Study
[0039] Disintegration time is an important indicator of orally disintegrating tablets. The orally disintegrating tablet of pregabalin of th...
Embodiment 2
[0048] Example 2. Pregabalin Dispersible Tablets
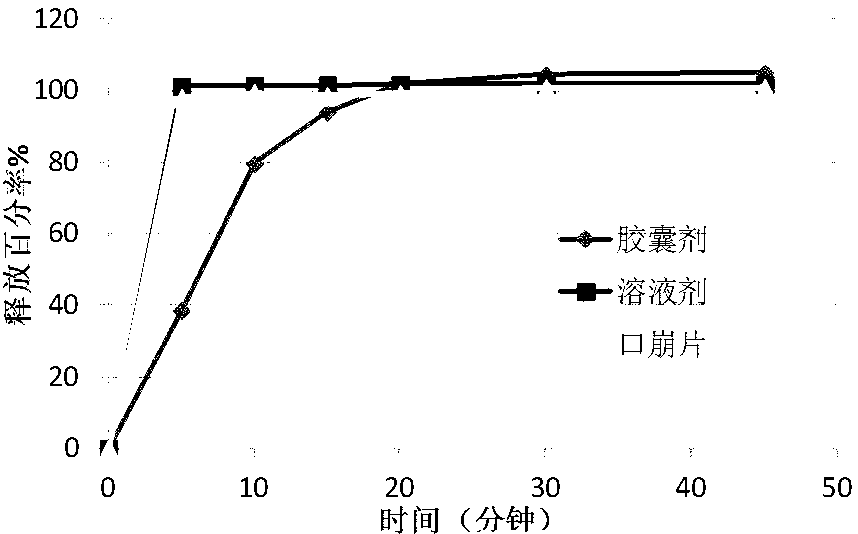
[0049] The composition of the pregabalin dispersible tablet is shown in Table 4. It has certain similarities with the commercially available pregabalin solution and can replace the pregabalin solution.
[0050] Table 4 Composition of Pregabalin Dispersible Tablets
[0051] .
[0052] Preparation:
[0053] 1. Pregabalin, sucralose, mannitol, citric acid, microcrystalline cellulose and crospovidone were mixed in a wet granulator;
[0054] 2. Add appropriate amount of water for granulation;
[0055] 3. The obtained granules are dried and sieved;
[0056] 4. The sieved granules are mixed with other external phases (strawberry essence, mannitol, microcrystalline cellulose, crospovidone, silicon dioxide and sodium stearyl fumarate);
[0057] 5. The final mixture is compressed with a 12mm die to obtain tablets of the required hardness.
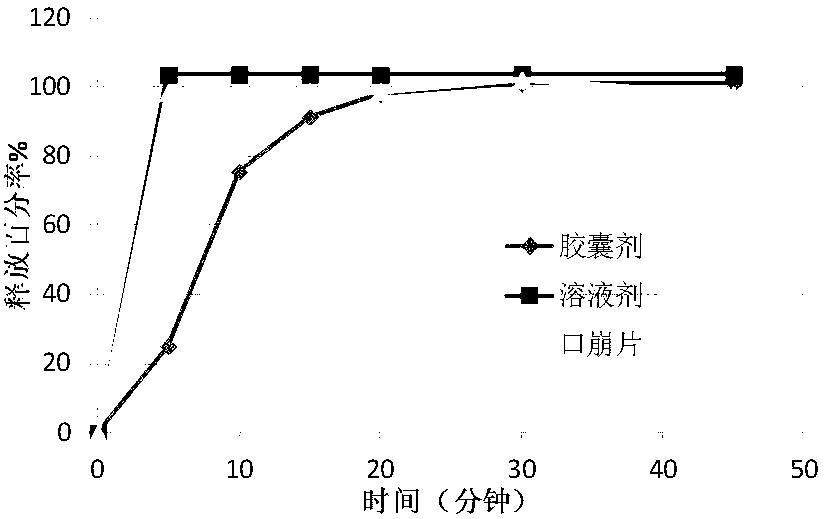
[0058] Disintegration Time Study
[0059]Disintegration time is also an important in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com