Sustained release tablet comprising pregabalin through two-phase release-controlling system
A technology of pregabalin and sustained-release tablets, which is applied in the directions of nervous system diseases, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., to achieve the effects of enhancing flotation and swelling.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to 34
[0035] Sustained-release tablets were prepared according to the ingredients and amounts shown in Tables 1 to 5. The amounts in Tables 1 to 5 represent the weight (mg) of each ingredient in each tablet. Pregabalin and hydroxypropyl methylcellulose (Metolose TM 90SH-100,000cps SR, Shinetsu) into a high-speed stirrer, and then a binder solution (prepared by dissolving hydroxypropyl cellulose (HPC JF) in a 50% ethanol solution) was added thereto. The mixture was granulated by spinning at 250 rpm for 3 minutes. The resulting granules are dried and then screened with a milling machine. The resulting granules are mixed with polyethylene oxide (Polyox301 and / or Polyox303), and supplementary flotation agents (crospovidone or sodium starch glycolate), diluents (hydroxypropylcellulose or microcrystalline cellulose) or lubricants (colloidal silicon dioxide) for 5 minutes. Magnesium stearate was blended with the mixture for an additional 3 minutes, followed by compression to obtain ext...
experiment example 1
[0049] Experimental Example 1: In Vitro Dissolution Test
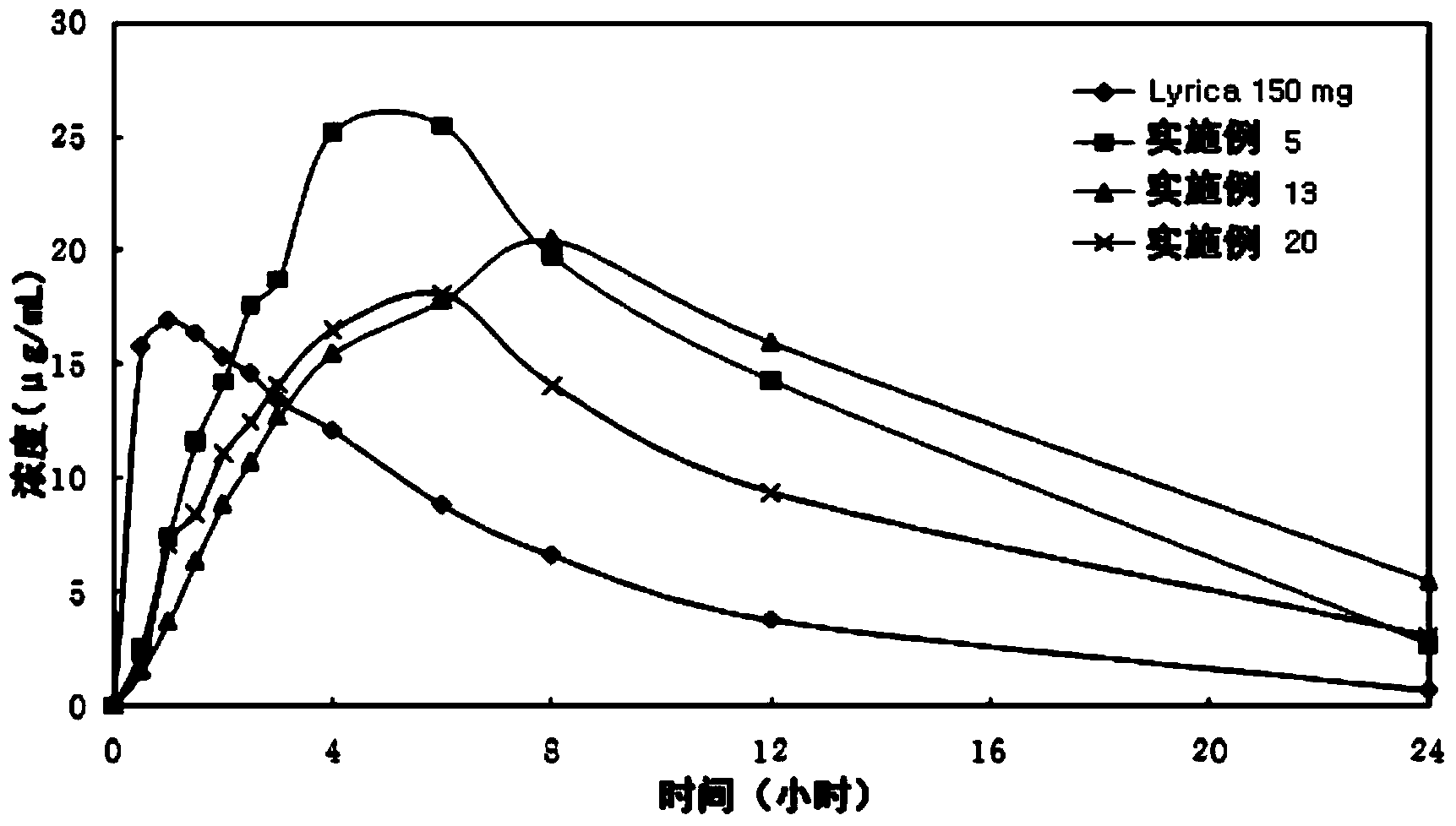
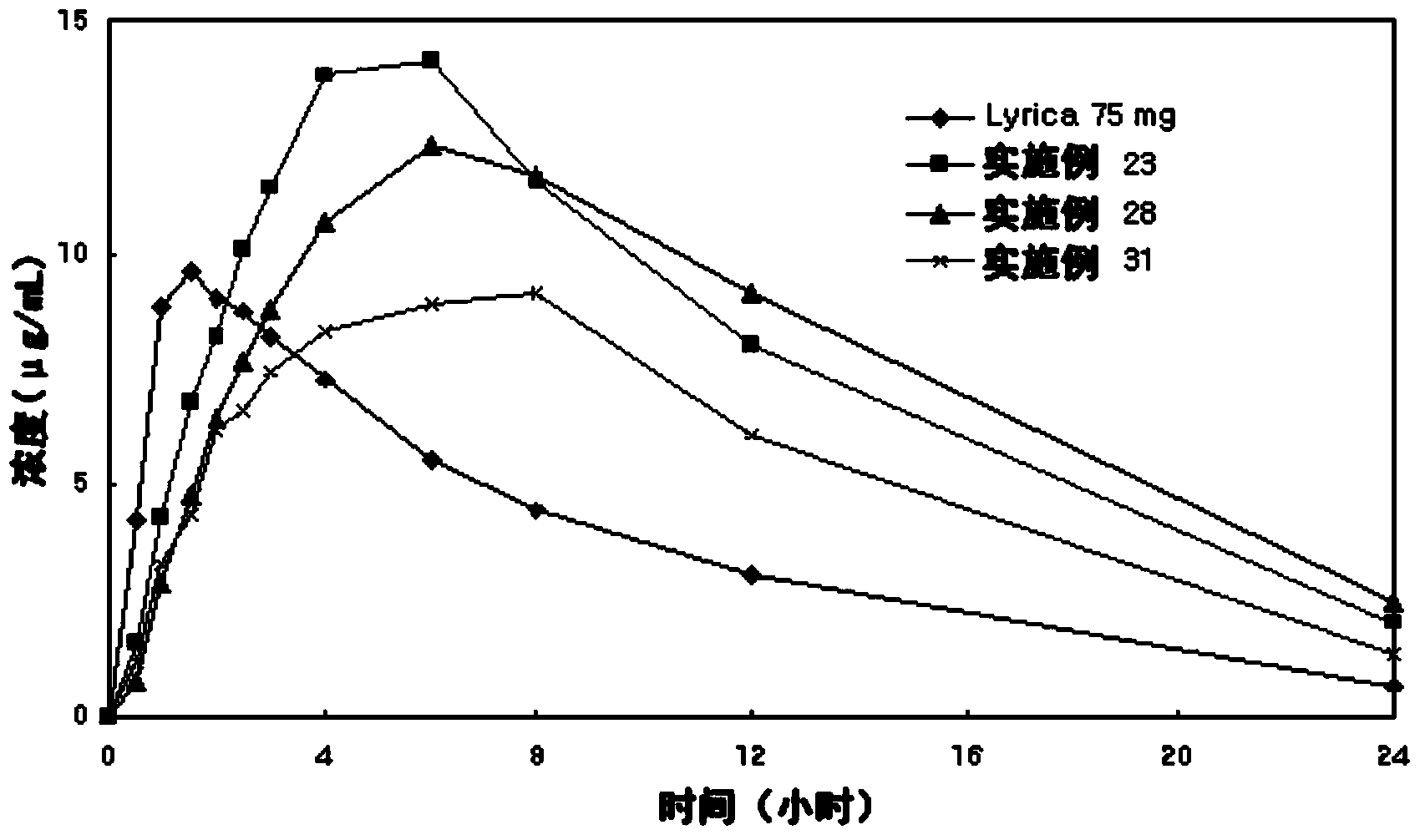
[0050] The tablets prepared in Examples 1 to 34 were subjected to a dissolution test according to "Dissolution Test 2 (Paddle Method)" of the Korean Pharmacopoeia. 900ml of 0.06N HCl solution was used as the dissolution medium, and the dissolution test was performed at 37±0.5°C with a paddle rotation speed of 50rpm. Aliquots were removed from the dissolution medium at 0.5, 1, 2, 3, 4, 6, 8, 12, 16 and 24 minutes. Each sample was analyzed by HPLC (at 210 nm) to calculate the dissolution rate. The results are shown in Tables 6-10. As shown in Tables 6 to 10,
[0051] Tablets prepared according to the present invention exhibit an excellent sustained release dissolution profile.
[0052]
[0053]
[0054]
[0055]
[0056]
[0057]
[0058]
[0059]
[0060]
[0061]
[0062]
[0063]
experiment example 2
[0064] Experimental Example 2: Measurement of floating-initiation time and floating-maintenance time
[0065] The tablets prepared in the above examples were subjected to a dissolution test according to "Dissolution Test 2 (Paddle Method)" of the Korean Pharmacopoeia. 900ml of 0.06N HCl solution was used as the dissolution medium, and the dissolution test was performed at 37±0.5°C with a paddle rotation speed of 50rpm. The floating lag time was measured, followed by the maintenance of floating time, up to 24 hours. The results are shown in Tables 11 to 13. As shown in Tables 11 to 13, the tablets prepared according to the present invention started to float within 30 minutes; and each tablet maintained floating for at least 24 hours.
[0066]
[0067]
[0068]
[0069]
[0070]
[0071]
[0072] floating time
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com