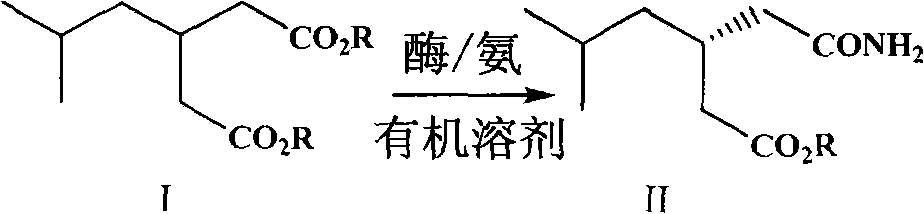
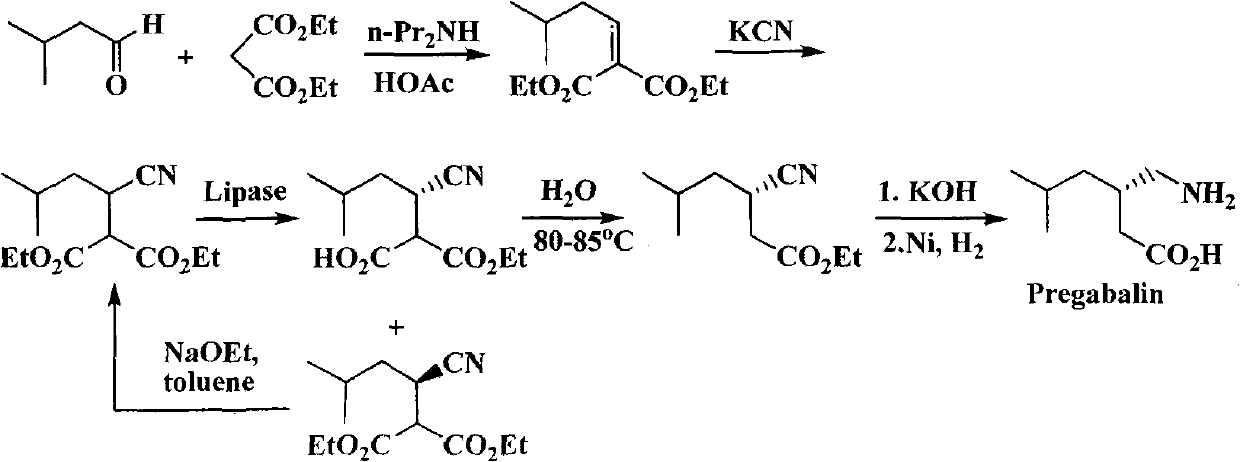
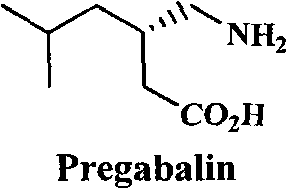Preparation of pregabalin chiral intermediate with bio-enzyme method
A chiral intermediate, pregabalin technology, applied in the field of pregabalin chiral intermediate preparation by biological enzymatic method, can solve the problems of low optical purity of pregabalin product, many reaction steps, difficult industrialization, etc., to achieve simple and more Synthesis, low cost, effect of reducing reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Add 0.05g of diethyl isobutylglutarate, 0.02g of ammonium carbamate, 1mL of tert-butanol, and 0.02g of biological enzymes into a 2mL centrifuge tube in sequence, seal it and place it in a shaker at 28°C and 180rpm for 66h . After completion of the reaction, centrifuge at 12,000 rpm for 10 min to remove enzymes and solid particles, and perform chromatographic detection and analysis. The conversion rate of the substrate is 98.41%, and the ee value of the product is 99.12%.
Embodiment 2
[0041] Example 2; (S)-3-(carbamoylmethyl)-5-methylhexanoic acid ethyl ester
[0042] Add 0.05g of diethyl isobutylglutarate, 0.02g of ammonium carbamate, 1mL of n-hexane, and 0.02g of biological enzyme into a 2mL centrifuge tube, seal it and place it in a shaker at 28°C and 180rpm to react for 66h. After completion of the reaction, centrifuge at 12000 rpm for 10 min to remove enzymes and solid particles. According to chromatographic analysis, the conversion rate of the substrate is 75.19%, and the ee value of the product is 98.35%.
Embodiment 3
[0043] Example 3: (S)-3-(carbamoylmethyl)-5-methylhexanoic acid ethyl ester
[0044] Add 0.05g of diethyl isobutylglutarate, 0.02g of ammonium carbamate, 1mL of isopropyl ether, and 0.02g of biological enzyme into a 2mL centrifuge tube in turn, seal it and place it in a shaker at 28°C and 180rpm to react for 66h . After completion of the reaction, centrifuge at 12000 rpm for 10 min to remove enzymes and solid particles, and perform chromatographic detection and analysis. The conversion rate is 66.57%, and the ee value of the product is 97.83%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com