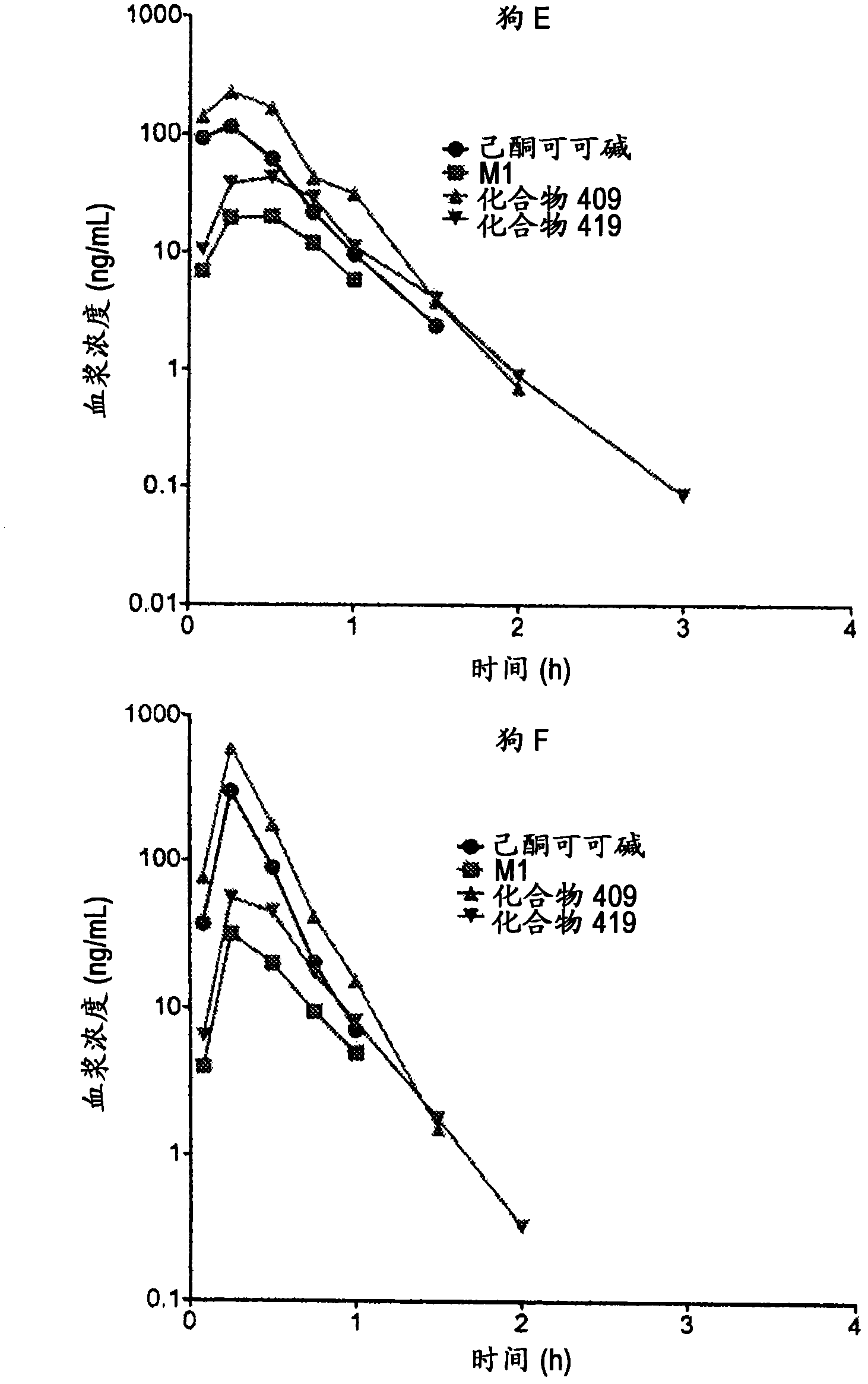
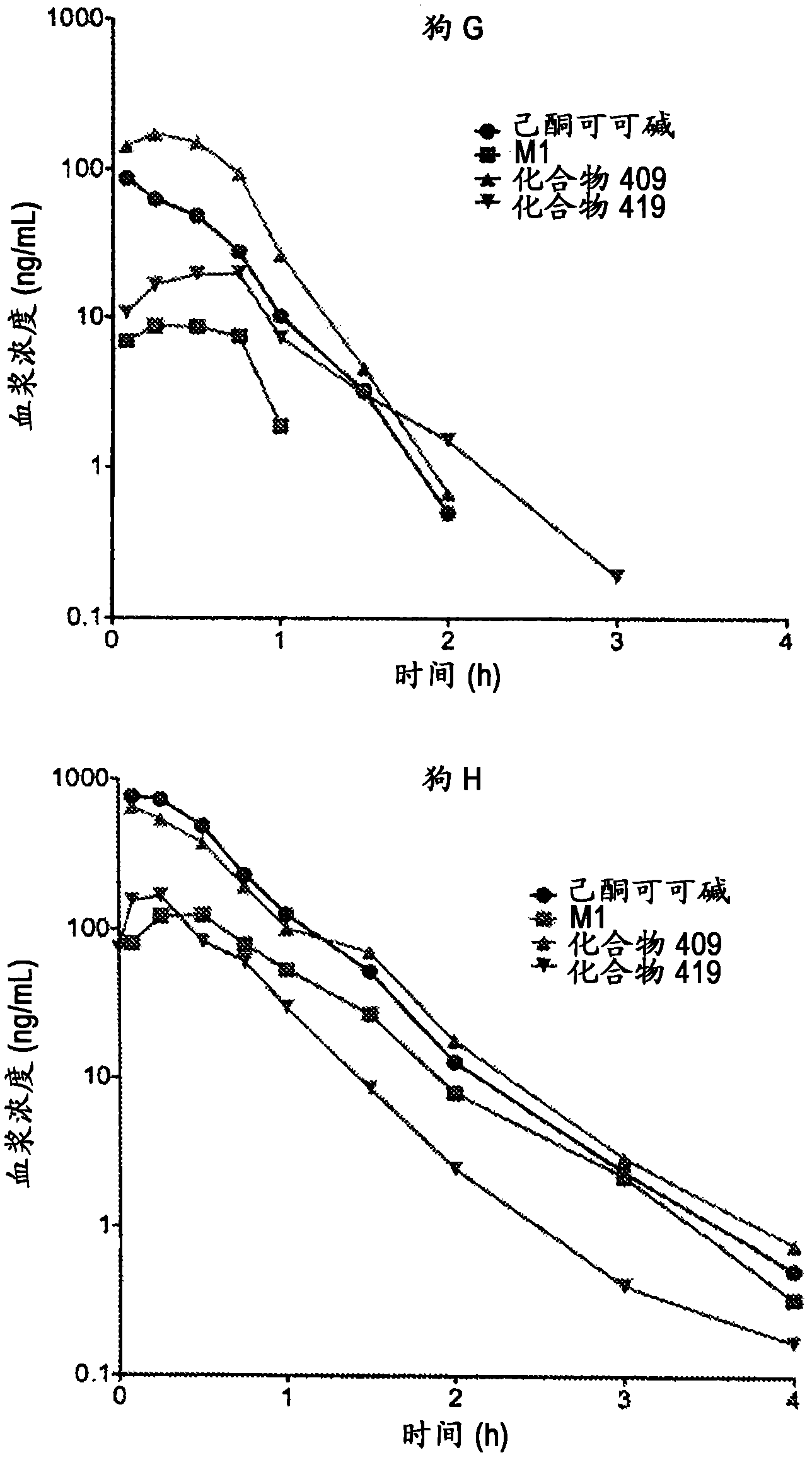
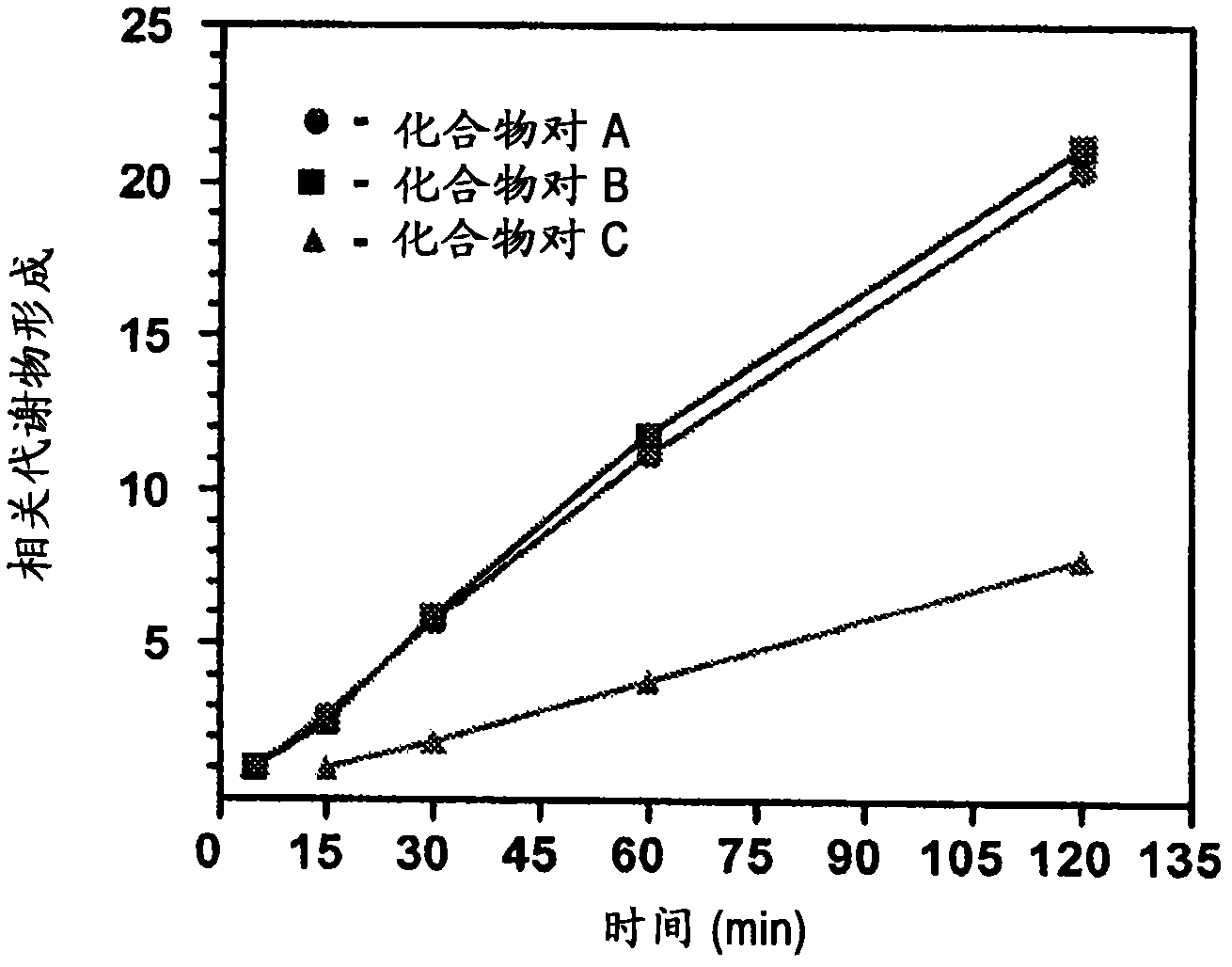
Substituted xanthine derivatives
A compound and composition technology, applied in the field of substituted xanthine derivatives, can solve problems such as unpredictable effects on drug metabolism properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
other Embodiment approach
[0118] Other embodiments provide compounds of formula B, wherein Y 1 and Y 2 Together with the carbon they are attached to form C=O. On the one hand, R 5 is deuterium. On the other hand, Z 3 ,Z 4 and Z 5 each is hydrogen and R 5 is deuterium. On the other hand, Z 3 ,Z 4 and Z 5 Each is deuterium and R 5 is deuterium. On the other hand, R 1 and R 2 Each is - CD 3 . On the other hand, R 1 and R 2 Each is - CD 3 and R 5 is deuterium. On the other hand, R 1 and R 2 Each is - CD 3 , and Z 3 ,Z 4 and Z 5 Each is deuterium. On the other hand, R 1 and R 2 Each is - CD 3 , and Z 3 ,Z 4 and Z 5 Each is deuterium and R 5 is deuterium. On the other hand, R 1 and R 2 Each is - CD 3 , and Z 3 ,Z 4 and Z 5 Each is hydrogen. On the other hand, R 1 and R 2 Each is - CD 3 , and Z 3 ,Z 4 and Z 5 each is hydrogen and R 5 is deuterium.
other Embodiment approach
[0119] Other embodiments provide compounds of formula B, wherein Y 1 is OH and Y 2 is hydrogen or deuterium. On the one hand, R 5 is deuterium. On the other hand, Z 3 ,Z 4 and Z 5 each is hydrogen and R 5 is deuterium. On the other hand, Z 3 ,Z 4 and Z 5 Each is deuterium and R 5 is deuterium. On the other hand, R 1 and R 2 Each is - CD 3 . On the other hand, R 1 and R 2 Each is - CD 3 and R 5 is deuterium. On the other hand, R 1 and R 2 Each is - CD 3 , and Z 3 ,Z 4 and Z 5 Each is deuterium. On the other hand, R 1 and R 2 Each is - CD 3 , and Z 3 ,Z 4 and Z 5 Each is deuterium and R 5 is deuterium. On the other hand, R 1 and R 2 Each is - CD 3 , and Z 3 ,Z 4 and Z 5 Each is hydrogen. On the other hand, R 1 and R 2 Each is - CD 3 , and Z 3 ,Z 4 and Z 5 each is hydrogen and R 5 is deuterium.
[0120] Another embodiment provides a compound of formula B, wherein R 5 is deuterium.
[0121] Another embodiment provides a compound ...
Embodiment 1
[0339] Example 1: 3-methyl-7-(methyl-d 3 )-1-(5-oxohexyl)-1H-purine-2,6(3H,7H)-dione (compound 100) synthesis
[0340] Route 13: Preparation of Compounds 100 and 409
[0341]
[0342] Step 1: 3-Methyl-7-(methyl-d 3 )-1H-purine-2,6(3H,7H)-dione (51). 3-Methylxanthine 50 (5.0g, 30.1mmol, 1 equivalent) and powdered K 2 CO 3 (5.0 g, 36.0 mmol, 1.2 equiv) in DMF (95 mL) was heated to 60 °C as a suspension and iodomethane-d was added via syringe 3 (Cambridge Isotopes, 99.5 atomic % D, 2.2 mL, 36.0 mmol, 1.2 equiv). The resulting mixture was heated at 80 °C for 5 hours (h). The reaction mixture was cooled to room temperature (rt) and DMF was evaporated under reduced pressure. The crude residue was dissolved in 5% aqueous NaOH (50 mL), forming a dark yellow solution. use CH 2 Cl 2 The aqueous solution was washed three times (total 500 mL). The aqueous layer was acidified to pH 5 with acetic acid (6 mL), forming a brown precipitate. The mixture was cooled in an ice-water...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com