Pentoxifylline slow-release tablet and preparation method thereof
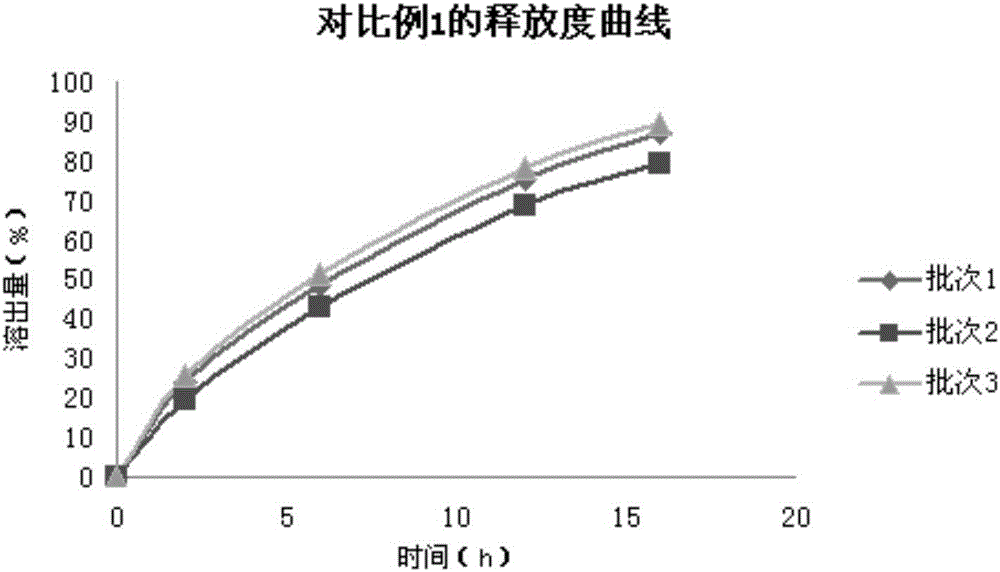
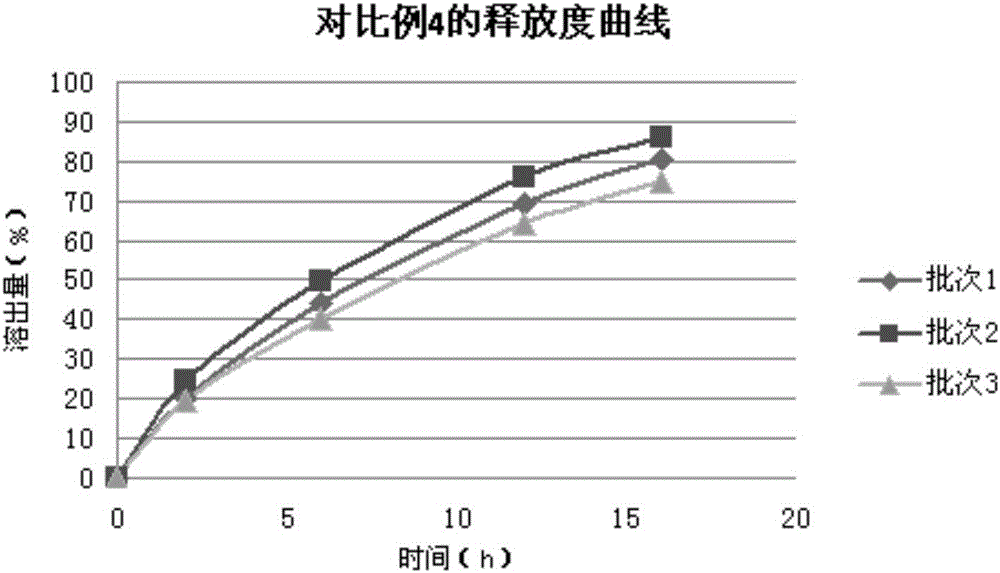
A technology of theobromine sustained-release tablets and pentoxifylline, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, and medical preparations containing active ingredients, etc. Sustained-release tablets have problems such as poor reproducibility of drug release, and achieve good sustained-release effects, good product quality uniformity, and good reproducibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
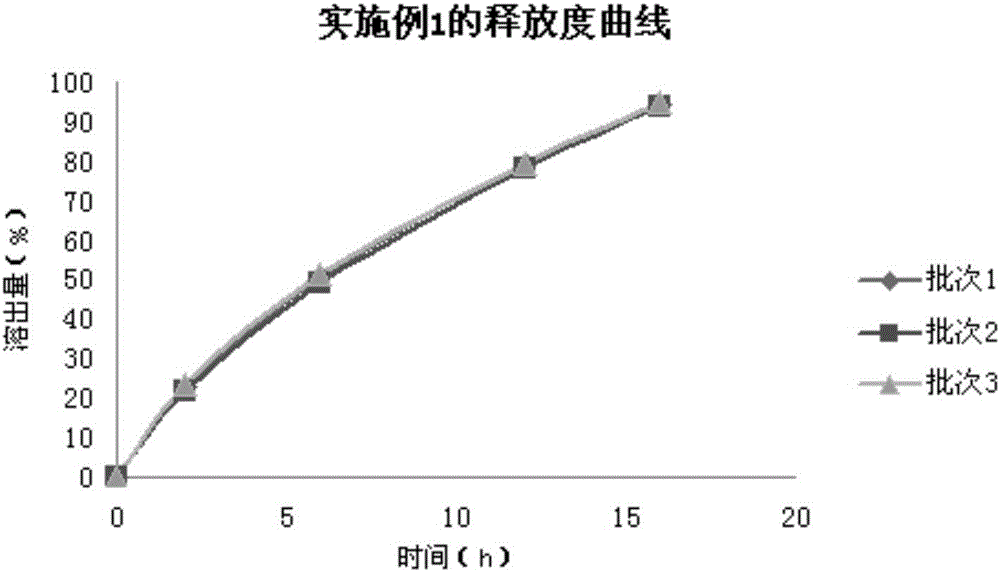
Embodiment 1
[0035] Embodiment 1 The preparation of pentoxifylline sustained-release tablets of the present invention
[0036]
[0037] Preparation Process:
[0038] 1) Add 90 g of pentoxifylline into molten glyceryl behenate, stir to disperse the drug evenly, cool in an ice bath for 6 hours, pulverize, and granulate with a 60-mesh sieve to obtain Granule 1;
[0039]2) Add the remaining pentoxifylline and polyoxyethylene into the granulator and stir at a speed of 100 rpm, and pre-mix for 5 minutes; then add 5% povidone K30 in 95% ethanol solution to make a soft material, and stir at 180 rpm, granulation for 10 minutes, granulation with a 20-mesh sieve, transfer the wet granules to a boiling dryer for drying, and the inlet air temperature is 60°C to obtain granules 2;
[0040] 3) Mix granule 1, granule 2, and magnesium stearate in a mixer for 10 minutes, the rotating speed is 12 rpm, and compressed into tablets (each tablet contains 0.4 g of pentoxifylline), and the tablet core hardness...
Embodiment 2
[0042] Embodiment 2 Preparation of Pentoxifylline Sustained-release Tablets of the Present Invention
[0043]
[0044] Preparation Process:
[0045] 1) Add 70 g of pentoxifylline into molten glyceryl behenate, stir to disperse the drug evenly, cool in an ice bath for 8 hours, pulverize, and sieve with a 60-mesh sieve to obtain Granule 1;
[0046] 2) Add the remaining pentoxifylline and polyoxyethylene into the granulator and stir at a speed of 105 rpm, pre-mix for 4 minutes; then add 5% povidone K30 in 95% ethanol solution to make a soft material, stir at a speed of 185 rpm, granulation for 12 minutes, granulation with a 20-mesh sieve, transfer the wet granules to a boiling dryer for drying, the inlet air temperature is 55°C, and granule 2 is obtained;
[0047] 3) Mix granule 1, granule 2, and magnesium stearate in a mixer for 11 minutes at a speed of 10 rpm, and press into tablets (each tablet contains 0.4 g of pentoxifylline), and the hardness of the tablet core is contr...
Embodiment 3
[0049] Embodiment 3 Preparation of Pentoxifylline Sustained-release Tablets of the Present Invention
[0050]
[0051] Preparation Process:
[0052] 1) Add 110 g of pentoxifylline into molten glyceryl behenate, stir to disperse the drug evenly, cool in an ice bath for 5 hours, pulverize, and granulate with a 60-mesh sieve to obtain Granule 1;
[0053] 2) Add the remaining pentoxifylline and polyoxyethylene into the granulator and stir at a speed of 95 rpm, and pre-mix for 3 minutes; then add 5% povidone K30 in 95% ethanol solution to make a soft material, and stir 190 rpm, granulation for 11 minutes, granulation with a 20-mesh sieve, transfer the wet granules to a boiling dryer for drying, and the inlet air temperature is 50°C to obtain granules 2;
[0054] 3) Mix granule 1, granule 2, and magnesium stearate in a mixer for 15 minutes at a speed of 11 rpm, and press into tablets (each tablet contains 0.4 g of pentoxifylline), and the hardness of the tablet core is controlle...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com