Pentoxifylline sustained release tablets and preparation thereof
A technology of pentoxifylline and theobromine, which is applied in the field of sustained-release tablets, can solve the problems of poor stability and frequent medication, and achieve the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Pentoxifylline 100g
[0015] Hypromellose K4M 100g
[0016] PEG2000 20g
[0017] Lactose 10g
[0019]
[0020] Makes 1000 pieces
[0021] Pass pentoxifylline, PEG2000, hypromellose K4M and lactose through a 40-mesh sieve, mix evenly, prepare a soft material with 5% povidone K30 (PVP K30) alcohol solution, and granulate with a 16-mesh sieve. The wet granules are dried at 40°C for 2 hours, sieved with a 14-mesh sieve, added with prescribed amount of magnesium stearate, mixed evenly, and then compressed into tablets.
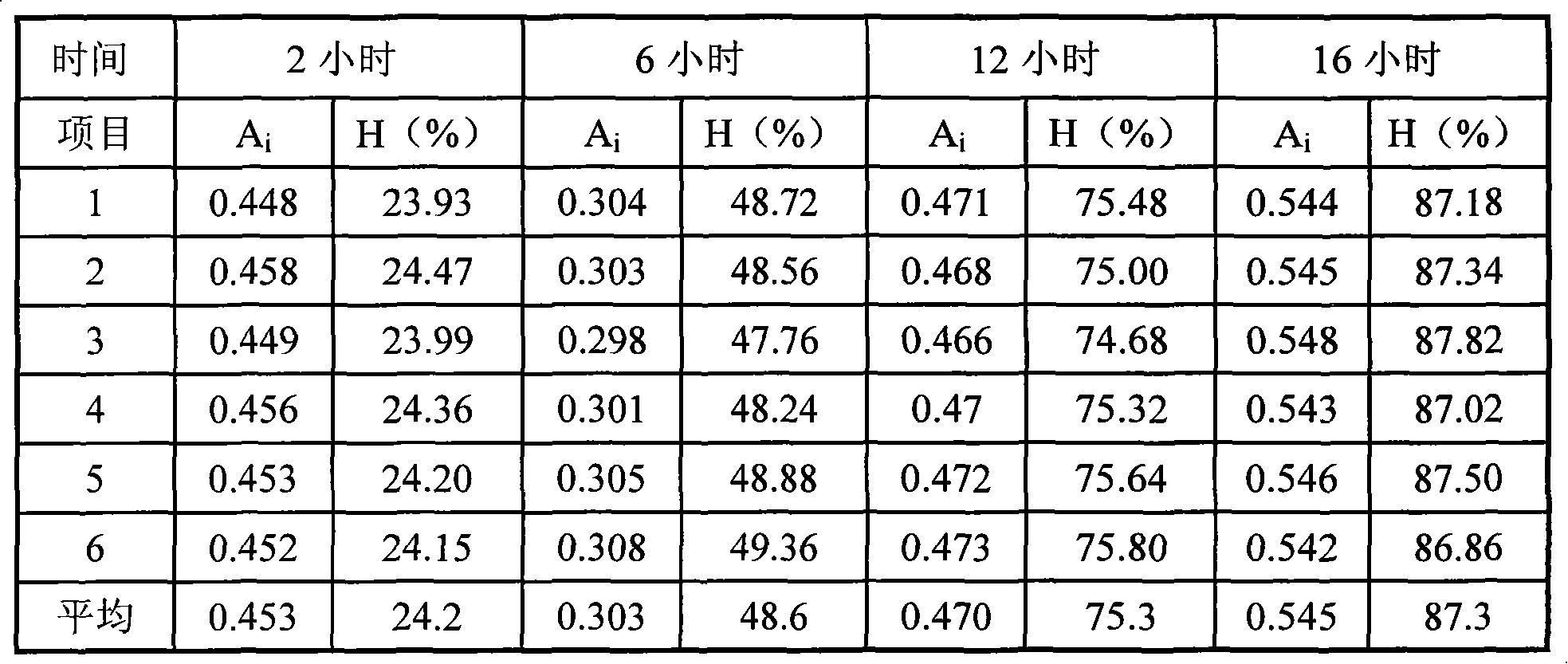
[0022] Determination of release: get this product, according to release assay (Chinese Pharmacopoeia in 2005 edition two appendix X D first method), adopt the device of dissolution assay (Chinese Pharmacopoeia in 2005 edition two appendix X C) second method, with hydrochloric acid Solution (9 → 1000) 900ml is the solvent, the speed is 50 revolutions per minute, oper...
Embodiment 2
[0028] Pentoxifylline 200g
[0029] Hypromellose K4M 65g
[0030] Hypromellose K15M 40g
[0031] PEG4000 20g
[0032] Microcrystalline Cellulose 15g
[0033] Micronized silica gel 1g
[0034]
[0035] Makes 1000 pieces
[0036] Pass the pentoxifylline and the excipients in the prescription (except micronized silica gel) through a 60-mesh sieve, mix evenly, prepare a soft material with 5% povidone K30 (PVP K30) ethanol solution, and granulate with a 16-mesh sieve. The wet granules were dried at 40°C for 2 hours, sieved with a 14-mesh sieve, added the prescribed amount of micro-powdered silica gel, mixed evenly, and then pressed into tablets.
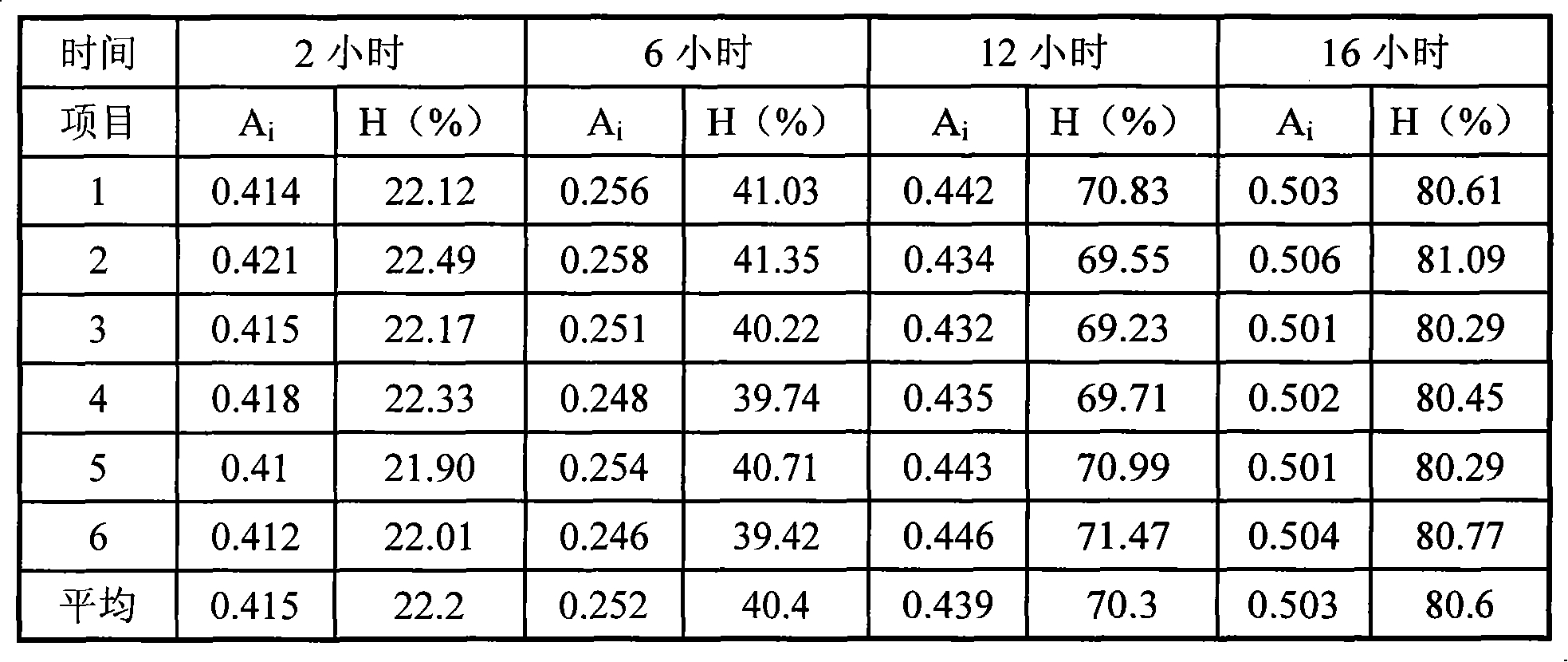
[0037] The released amount of each tablet of the sample in this example is 22.2%, 40.4%, 70.3% and 80.6% of the labeled amount at 2, 6, 12 hours and 16 hours respectively.
[0038] test results
[0039]
Embodiment 3
[0041] Pentoxifylline 400g
[0042] Hypromellose K4M 75g
[0043] Hypromellose K100M 20g
[0044] PEG6000 20g
[0045] Starch 10g
[0047]
[0048] Makes 1000 pieces
[0049] Pass pentoxifylline and the auxiliary materials in the prescription (except talcum powder) through a 80-mesh sieve, mix evenly, prepare a soft material with 5% starch slurry, and granulate with a 16-mesh sieve. The wet granules were dried at 40°C for 2 hours, sieved with a 14-mesh sieve, added the prescribed amount of talcum powder, mixed evenly, and then pressed into tablets.
[0050] The released amount of each tablet of the sample in this example is 17.3%, 36.9%, 69.3% and 78.6% of the labeled amount at 2, 6, 12 hours and 16 hours respectively.
[0051] test results
[0052]
[0053]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com