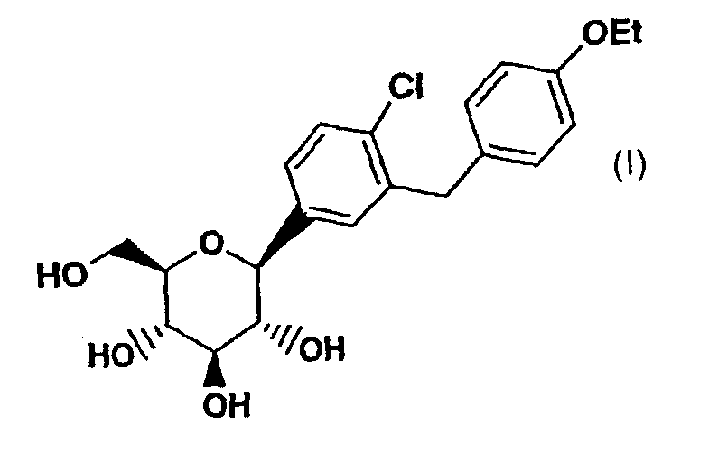
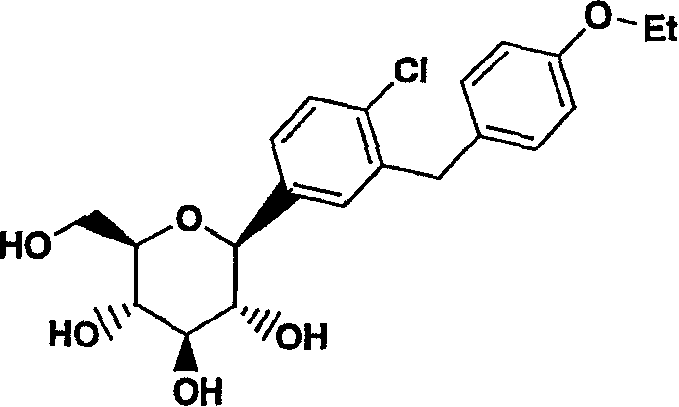
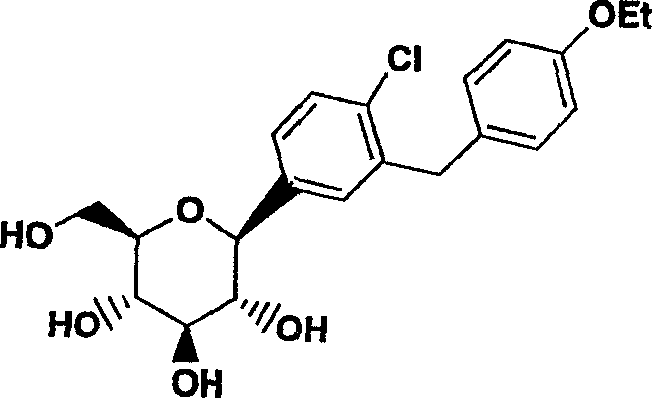
C-aryl glucoside SGLT2 inhibitors and method
A technology of inhibitors and reagents, applied in the direction of carbocyclyl sugars, sugar derivatives, pharmaceutical formulations, etc., can solve the problems of exacerbated peripheral insulin resistance, low blood sugar, glucose absorption damage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0196]
[0197] A. 5-Bromo-2-chloro-4'-ethoxybenzophenone
[0198] Commercially available 5-bromo-2-chlorobenzoic acid (250 g, 1.06 mol) was added to 450 ml of CH containing oxalyl chloride (1.1 mol) 2 Cl 2 , stirred to make a suspension, and then added 1.5ml DMF to it. Once the evolution of gas ceased, the reaction was stirred overnight, then the volatiles were removed under vacuum using a rotary evaporator. Dissolve the crude 5-bromo-2-chlorobenzoyl chloride in 200 ml CH 2 Cl 2 , the yellow solution was transferred to a 2 L three-necked flask equipped with an overhead stirrer and an internal thermometer. The stirred mixture was cooled to -3°C, then phenetole (130 g, 1.08 mol) was added. Gradually add AlCl over a period of 30 min via the solids addition funnel 3 (140 g, 1.07 mol) to ensure that the temperature does not exceed 4°C. After adding 60% AlCl 3 A large amount of HCl gas which then started to be evolved was absorbed by the stirred concentrated NaOH solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com