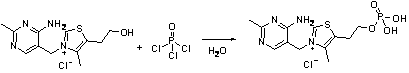Method for synthesizing benfotiamine
A synthesis method and benfotiamine technology are applied in the field of organic compound preparation process improvement, can solve problems such as increasing process steps, increasing production cycle, reducing production efficiency and the like, and achieve the effects of high purity, improved production efficiency and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
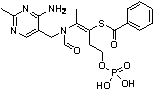
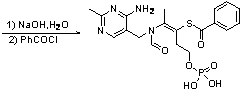
[0026] Example 1: Add 15.33g (0.1mol) of phosphorus oxychloride to 10.8mL of water, place it in an ice bath and stir for 0.5 hours, add 26.53g (0.1mol) of thiamine in batches, and then heat to 50°C and stir for 2 After hours, cool to room temperature to obtain phosphorthiamine solution. The HPLC content of phosphorthiamine is 91.36%. Use 15% NaOH solution to adjust the pH of the solution to 8-9, add 28.11g (0.2mol) benzoyl chloride, at 0- Stir at 5°C and monitor the pH of the reaction solution to stabilize the pH value of the solution. When the pH of the reaction solution no longer changes, continue to stir and react for 1 hour, adjust the pH of the solution to 3.5-4.0, and filter with suction to obtain 33.58 g of phenylphosphonium. Thiamine is a white solid. The yield was 71.9%.
[0027] MP:164-165℃; H 1 NMR(400MHz , CDCl 3 ): 2.18 (s, 3H), 2.56 (s, 3H), 2.58 (t, J =6.7, 2H), 4.33 (t, J =6.7, 2H), 4.83 (s, 2H), 7.44 (m, 2H), 7.57 (dd, J =7.3, J =1.5,1H), 7.60 (m, 2H), 7.70 (s,...
Embodiment 2
[0028] Example 2: Add 15.33g (0.1mol) of phosphorus oxychloride to 7.2mL water, place in an ice bath and stir for 0.5 hours, add 21.23g (0.08mol) of thiamine in batches, and then heat to 60°C and stir for 2 After hours, cool to room temperature to obtain phosphorthiamine solution. The HPLC content of phosphorthiamine is 92.37%. Use 15% NaOH solution to adjust the pH of the solution to 8-9, add 28.11g (0.2mol) benzoyl chloride, Stir at 5°C and monitor the change of the pH of the reaction solution to stabilize the pH of the solution. When the pH of the reaction solution no longer changes, continue to stir and react for 1 hour, adjust the pH of the solution to 3.5-4.0, and filter with suction to obtain 27.69 g of phenphos Thiamine is a white solid. The yield was 74.2%.
[0029] MP:164-165℃;H 1 NMR(400MHz , CDCl 3 ): 2.18 (s, 3H), 2.56 (s, 3H), 2.58 (t, J =6.7, 2H), 4.33 (t, J =6.7, 2H), 4.83 (s, 2H), 7.44 (m, 2H), 7.57 (dd, J =7.3, J =1.5,1H), 7.60 (m, 2H), 7.70 (s, 1H), 8.67 (s, ...
Embodiment 3
[0030] Example 3: Add 15.33g (0.1mol) of phosphorus oxychloride to 3.6mL of water, place it in an ice bath and stir for 0.5 hours, add 15.92g (0.06mol) of thiamine in batches, then heat to 70°C and stir for 2 After hours, cool to room temperature to obtain phosphorthiamine solution. The HPLC content of phosphorthiamine is 93.23%. Adjust the pH of the solution to 8-9 with 15% NaOH solution. Add 28.11g (0.2mol) of benzoyl chloride, Stir at 5°C and monitor the pH of the reaction solution to stabilize the pH value of the solution. When the pH of the reaction solution no longer changes, continue to stir and react for 1 hour, adjust the pH of the solution to 3.5-4.0, and filter with suction to obtain benfotiamine White solid 23.71g. The yield was 84.7%.
[0031] MP:164-165℃;H 1 NMR(400MHz , CDCl 3 ): 2.18 (s, 3H), 2.56 (s, 3H), 2.58 (t, J =6.7, 2H), 4.33 (t, J =6.7, 2H), 4.83 (s, 2H), 7.44 (m, 2H), 7.57 (dd, J =7.3, J =1.5,1H), 7.60 (m, 2H), 7.70 (s, 1H), 8.67 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com