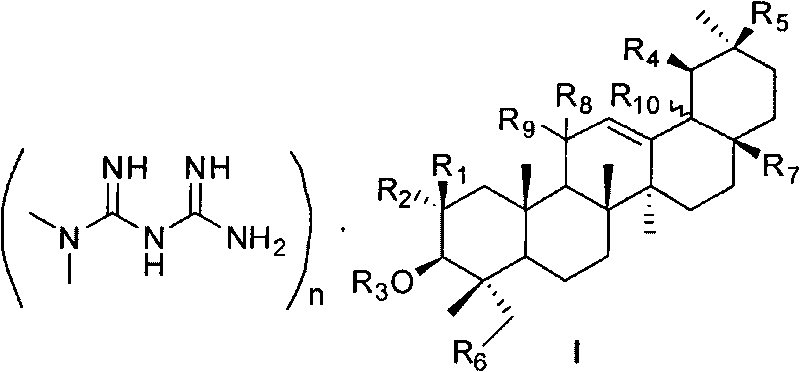
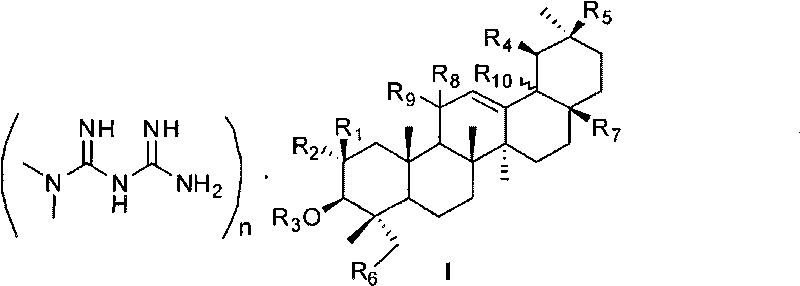
Pentacyclic triterpene and melbine salt of derivative thereof, preparation method and medical application of pentacyclic triterpene
A technology of metformin salt and pentacyclic triterpene, which is applied in the fields of natural medicine and medicinal chemistry, and can solve problems such as high water solubility, side effects, and limited application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] 3-O-Succinyl Glycyrrhetinic Acid Hemimetformin Salt
[0084]
[0085] Dissolve 113mg (0.88mmol, 1eq) of metformin in 15ml of methanol, then add 500mg (0.88mmol) of 3-O-succinylglycyrrhetinic acid, and stir at room temperature until dissolved. After 3 hours, filter to remove insoluble impurities, then put it into the refrigerator to cool and crystallize, filter with suction, and dry to obtain a white solid product which is 3-O-succinylglycyrrhetinic acid hemimetformin salt. M.p. 256-258°C. 1 H NMR (DMSO-d 6 , 300MHz) δ0.75(s, 3H), 0.82(s, 6H), 1.04(s, 3H), 1.06(s, 6H), 1.36(s, 3H), 2.34-2.47(m, 4H), 2.92 (s, 3H), 4.40-4.45 (m, 1H), 5.41 (s, 1H); Anal. Calcd for C 72 h 111 o 14 N 5 0.5H 2 O: C 67.57, H 8.82, N 5.47, Found: C 67.38, H 8.78, N 5.92.
Embodiment 2
[0087] 3-O-Succinyl Glycyrrhetinic Acid Monometformin Salt
[0088]
[0089] Dissolve 227mg (1.76mmol, 2eq) of metformin in 26ml of methanol, then slowly add 500mg (0.88mmol, 1eq) of 3-O-succinylglycyrrhetinic acid, stir at room temperature for 1h, heat up to 60°C to dissolve the reactant, filter, and filtrate Cooling and crystallization, suction filtration and drying, the white solid product obtained was 3-O-succinylglycyrrhetinic acid monometformin salt. M.p. 232-234°C. 1 H NMR (DMSO-d 6 , 300MHz) δ0.74(s, 3H), 0.82(s, 6H), 1.02(s, 3H), 1.04(s, 3H), 1.06(s, 3H), 1.36(s, 3H), 2.26-2.39 (m, 4H), 2.92 (s, 6H), 4.38-4.44 (m, 1H), 5.42 (s, 1H).
Embodiment 3
[0091] 18β-Glycyrrhizinate Monometformin Salt
[0092] Dissolve 314.76mg (2.44mmol, 2eq) of metformin in 25ml of methanol, then slowly add 1.02g (1.22mmol, 1eq) of 18β-glycyrrhizic acid, and stir at room temperature for 1h. When the temperature was raised to 70°C, the reactant still could not be completely dissolved, and methanol was added until the total amount was 40ml, and the reactant was dissolved. After 2 hours, the reaction was stopped, cooled and crystallized, filtered with suction, and dried to obtain a white solid product which was 18β-glycyrrhizic acid monometformin salt. M.P. 205-206°C. 1 H NMR (DMSO-d 6 +D 2 O, 300MHz) δ0.66(s, 3H), 0.70(s, 3H), 0.89(s, 3H), 0.97(s, 6H), 1.05(s, 3H), 1.27(s, 3H), 2.89( s, 6H), 4.30 and 4.33 (d, J = 6.4Hz, each 1H), 4.50 and 4.53 (d, J = 7.5Hz, each 1H), 5.38 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com