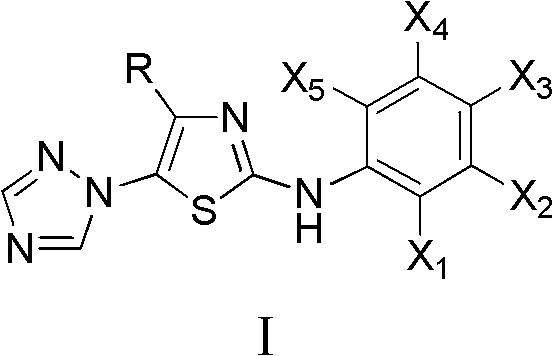
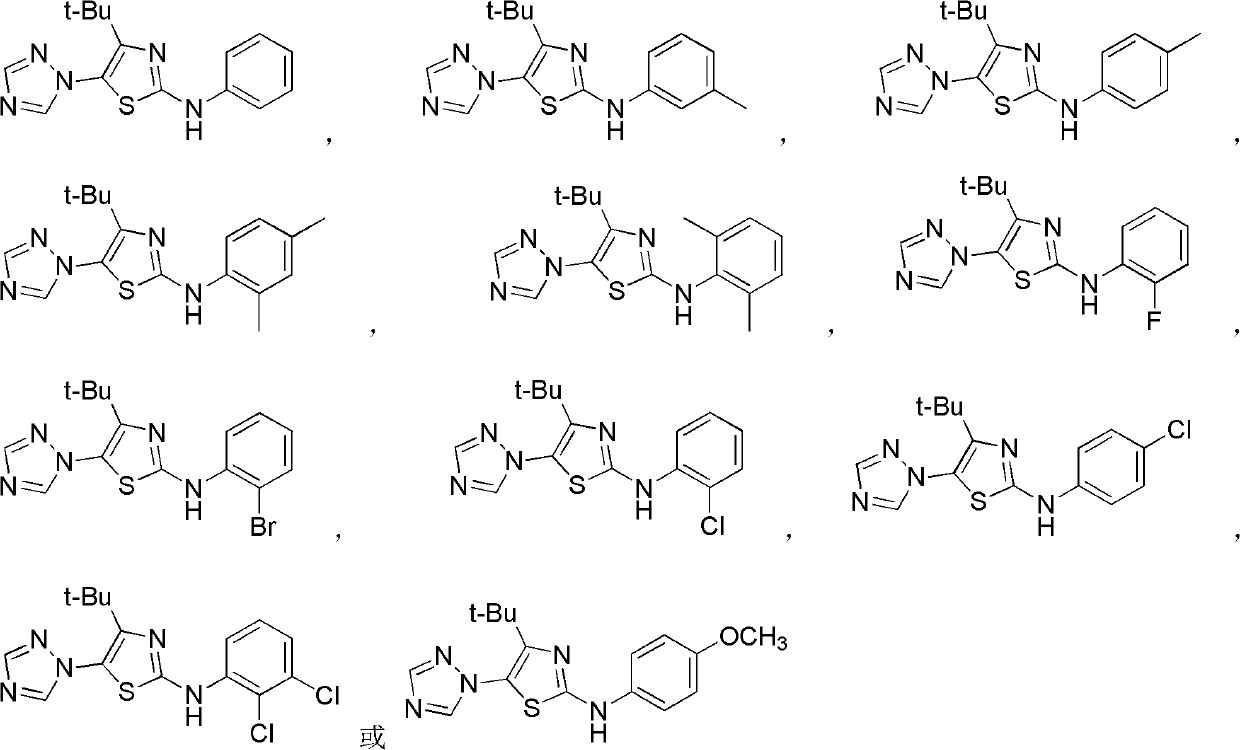
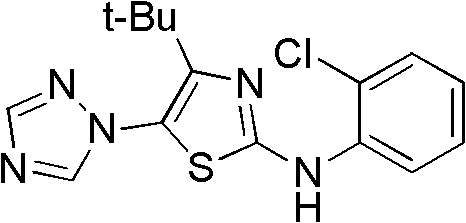
4-alkyl-2-arylamino-5-(1,2,4-triazole-1-group) thiazole and application thereof to preparation of medicaments for resisting cancer
A technology of arylamino and alkyl, which is applied in the field of new compounds and their preparation, and can solve the problems of no research and development reports in the application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0031] Preparation of Example 14-tert-butyl-2-phenylamino-5-(1,2,4-triazol-1-yl)thiazole and its hydrobromide
[0032]
[0033]3,3-Dimethyl-1-(1,2,4-triazol-1-yl)-1-bromo-2-butanone and phenylthiourea compounds are fed in a molar ratio of 1:1, in ethanol Heating to reflux reaction. After the reaction was completed, the solvent was distilled off under reduced pressure, washed with ethyl acetate, filtered, and dried to obtain 4-tert-butyl-2-phenylamino-5-(1,2,4-triazol-1-yl)thiazole hydrobromide Salt; hydrobromide was neutralized with ammonia water, extracted with ethyl acetate, distilled under reduced pressure, and recrystallized from ethanol to obtain 4-tert-butyl-2-phenylamino-5-(1,2,4-triazol-1-yl ) Thiazole, melting point 186-188°C. 1 H NMR (CDCl 3 , 400MHz), δ: 1.17(s, 9H, 3×CH 3 ), 1.83 (brs, 1H, NH), 7.10~7.40 (m, 5H, C 6 h 5 ), 8.10(s, 1H, C 2 h 2 N 3 3-H), 8.26(s, 1H, C 2 h 2 N 3 5-H).
Embodiment 24
[0034] Preparation of Example 24-tert-butyl-2-(3-methylanilino)-5-(1,2,4-triazol-1-yl)thiazole
[0035]
[0036] According to the method of Example 1, react 9.0h; Obtain 4-tert-butyl-2-(3-methylanilino)-5-(1,2,4-triazol-1-yl)thiazole; Melting point 98~ 100°C; 1 H NMR (CDCl 3 , 400MHz), δ: 1.16(s, 9H, 3×CH 3 ), 2.36(s, 3H, CH 3 ), 6.94 (d, J=8.0Hz, 1H, C 6 h 4 4-H), 7.12(d, J=6.4Hz, 1H, C 6 h 4 6-H), 7.13(s, 1H, C 6 h 4 2-H), 7.25(t, 1H, J = 7.2Hz, 1H, C 6 h 4 5-H), 8.08(s, 1H, C 2 h 2 N 3 3-H), 8.24(s, 1H, C 2 h 2 N 3 5-H).
Embodiment 34
[0037] Preparation of Example 34-tert-butyl-2-(4-methylanilino)-5-(1,2,4-triazol-1-yl)thiazole
[0038]
[0039] According to the method of Example 1, react 4.0h; Obtain 4-tert-butyl-2-(4-methylanilino)-5-(1,2,4-triazol-1-yl)thiazole; Melting point 147~ 150°C; 1 H NMR (CDCl 3 , 400MHz), δ: 1.17(s, 9H, 3×CH 3 ), 2.34(s, 3H, CH 3 ), 7.18 (d, J=8.8Hz, 2H, C 6 h 5 3,5-H), 7.22 (d, J=8.8Hz, 2H, C 6 h 5 2,6-H), 8.08(s,1H,C 2 h 2 N 3 3-H), 8.24(s, 1H, C 2 h 2 N 3 5-H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com