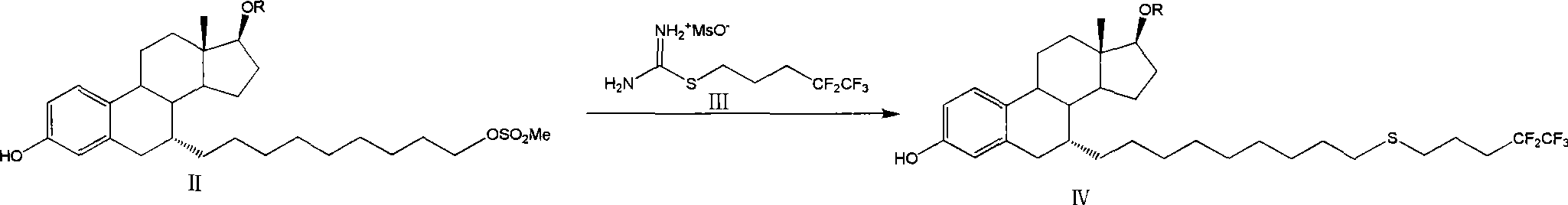
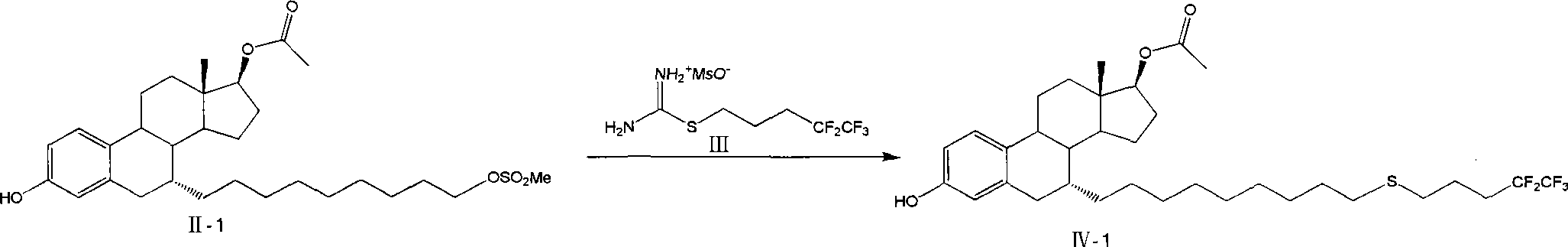
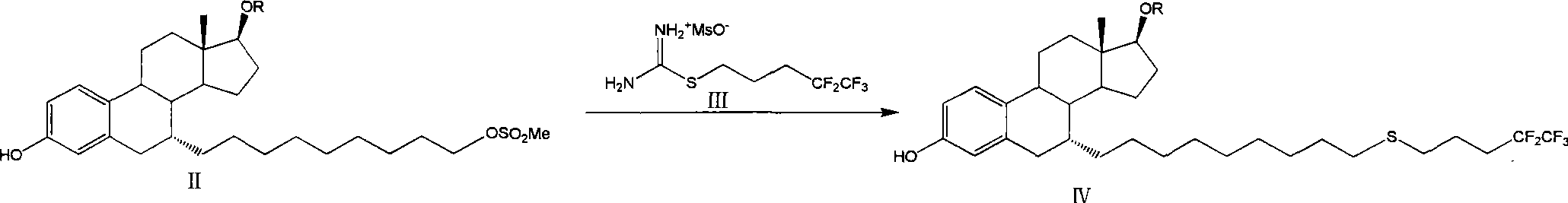
Preparation method of fulvestrant
A technology of fulvestrant and pentafluoropentyl, which is applied in the field of preparation of the anticancer drug fulvestrant, can solve problems such as the difficulty of fulvestrant synthesis, achieve easy post-processing, reduce production costs, and increase yields. rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation of embodiment 1 formula II-1 compound
[0042]
Embodiment 1-1
[0043] Example 1-1 Preparation of 9-bromononanol tert-butyldimethylsilyl ether
[0044]
[0045] Add 223 grams of 9-bromononanol, 205 grams of imidazole and 1 liter of dichloromethane into a 3-liter three-necked flask, and stir under nitrogen protection. At -15°C, 178 g of tert-butyldimethylsilyl chloride (dissolved in 200 ml of dichloromethane) was added dropwise, and the dropwise addition was completed within 1 hour. The reaction was carried out at temperature for 24 hours, and at room temperature for 24 hours. Wash with water 6 times after the reaction, each 1000 milliliters, then wash 2 times with saturated sodium chloride water. The organic phase was concentrated at 100°C to obtain 302 g of the title compound as a pale yellow viscous liquid.
Embodiment 1-2
[0046] Example 1-2 Preparation of 9-bromononanol tert-butyldimethylsilyl ether Grignard reagent
[0047]
[0048] Add 36 grams of metal magnesium chips and 2 liters of anhydrous tetrahydrofuran to the dried 3-liter three-neck flask under nitrogen protection, add a grain of iodine under stirring, the color of the solution turns yellowish brown, turn on the heating device, and add the above-mentioned Example 1 at 60°C The light yellow viscous liquid of -1 was refluxed while adding dropwise, and continued to reflux for 16 hours after the addition was completed, and then set aside for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com