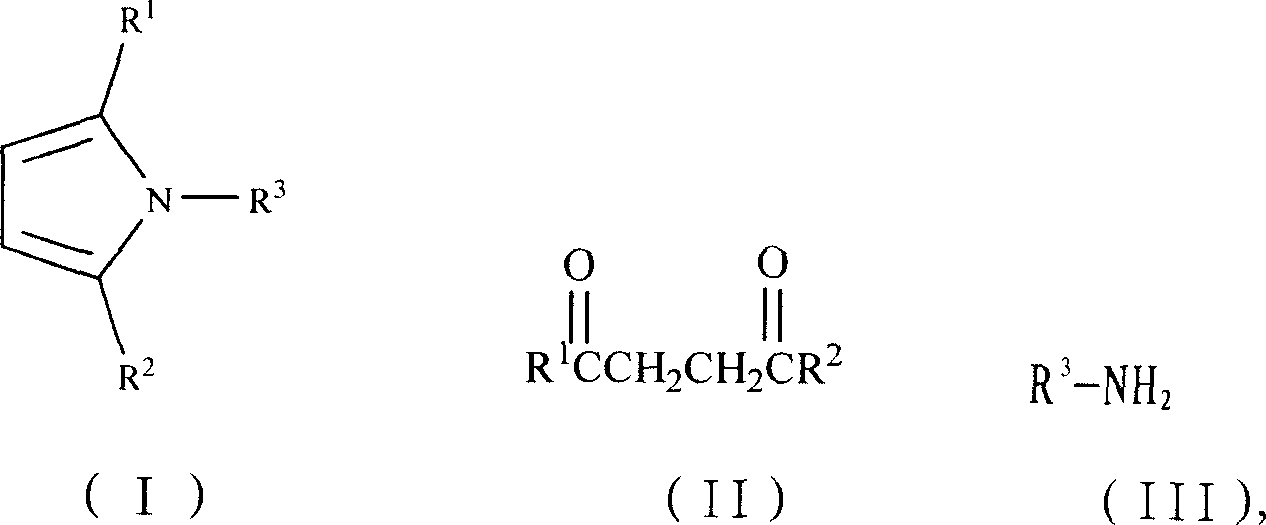Synthesis process of N-substituted pyrrole
A synthesis method and pyrrole technology, applied in organic chemistry and other directions, can solve the problems of complex catalyst preparation method, inability to recycle and apply, serious environmental pollution, etc., and achieve the effects of mild reaction conditions, no three wastes, and less dosage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
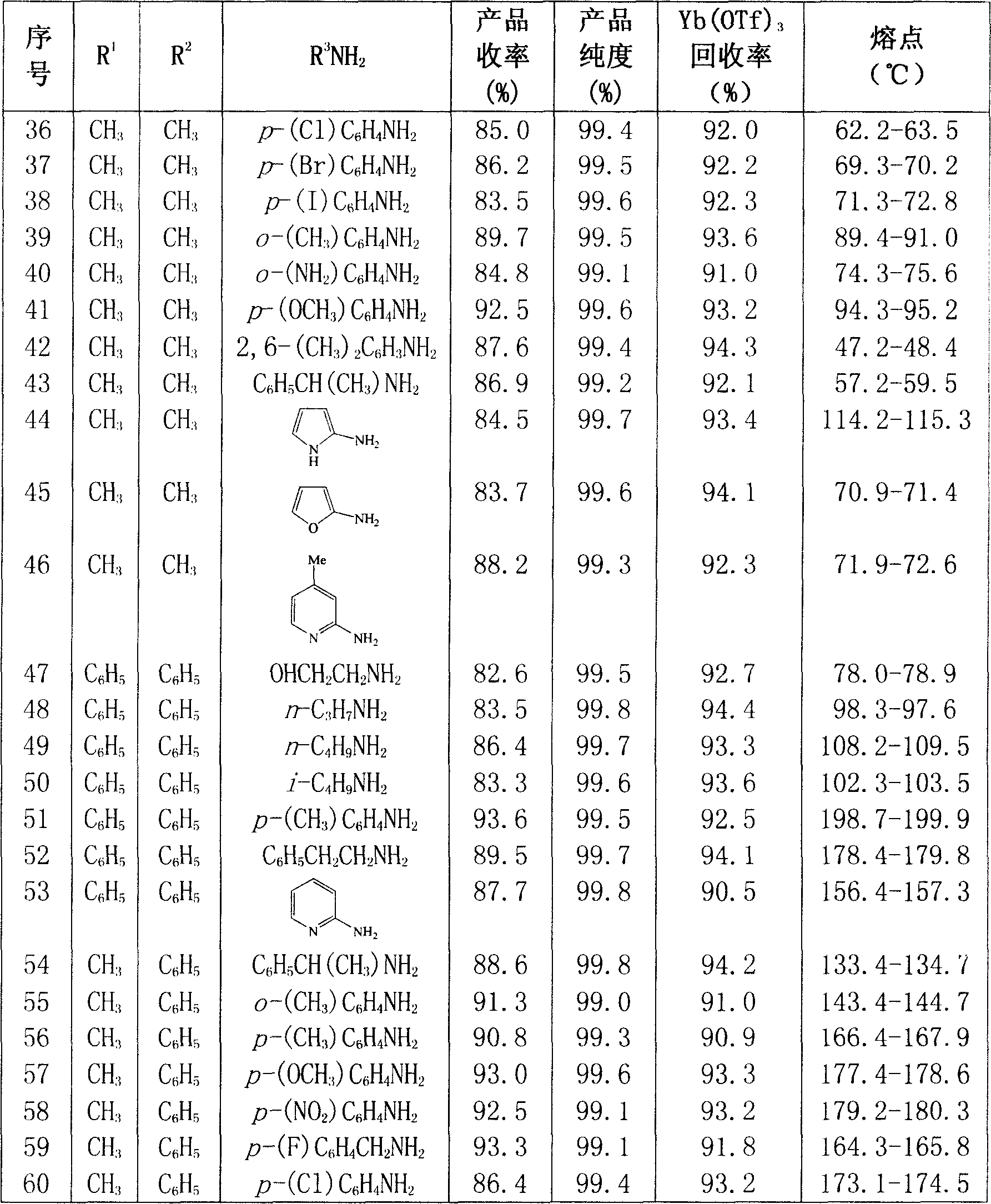
Image
Examples
Embodiment 1
[0024] The ratio of the feed materials of diketone, amine and ytterbium trifluoromethanesulfonate is 1:1:0.05, the diketone is 2,5-hexanedione, and the amine is aniline.
[0025] In a 50ml four-neck flask equipped with a thermometer, a reflux condenser and a mechanical stirrer, add 11.4g (0.1mol) of 2,5-hexanedione, 9.3g (0.1mol) of aniline, and 3.1g of ytterbium trifluoromethanesulfonate ( 5 mmol), heated to 50°C, and reacted for 30 minutes. Simultaneously, HPLC was followed and monitored (flow rate: 1.5 ml / min, acetonitrile: water: acetic acid=40:60:0.1). After the reaction was completed, cool to room temperature, filter and wash the filter cake with water, and collect the precipitated white solid by filtration again to obtain 15.2 g of the product N-phenyl-2,5-dimethylpyrrole, with a yield of 89%, a purity of 99.6%, and a melting point of 50.2~51.1℃, the recovery rate of ytterbium trifluoromethanesulfonate is 91.2%.
Embodiment 2
[0027] The ratio of feed materials of diketone, amine and zinc trifluoromethanesulfonate is 1:1:0.05, the diketone is 2,5-hexanedione, and the amine is aniline. 11.4 g (0.1 mol) of 2,5-hexanedione, 9.3 g (0.1 mol) of aniline, and 1.8 g (5 mmol) of zinc trifluoromethanesulfonate.
[0028] Others are the same as in Example 1 to obtain 13.7 g of the product N-phenyl-2,5-dimethylpyrrole, with a yield of 86%, a purity of 99.2%, and a melting point of 50.2 to 51.4°C. The reactant is used as a solvent for zinc trifluoromethanesulfonate Or 92.2% recovery in organic solvents.
Embodiment 3
[0030] The ratio of the feed materials of diketone, amine and lanthanum trifluoromethanesulfonate is 1:1:0.05, the diketone is 2,5-hexanedione, and the amine is aniline. 11.4 g (0.1 mol) of 2,5-hexanedione, 9.3 g (0.1 mol) of aniline, and 2.9 g (5 mmol) of lanthanum trifluoromethanesulfonate.
[0031] Others were the same as in Example 1 to obtain 15.0 g of the product N-phenyl-2,5-dimethylpyrrole with a yield of 88%, a purity of 99.0%, a melting point of 50.1-51.0°C, and a recovery rate of lanthanum trifluoromethanesulfonate of 94.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com