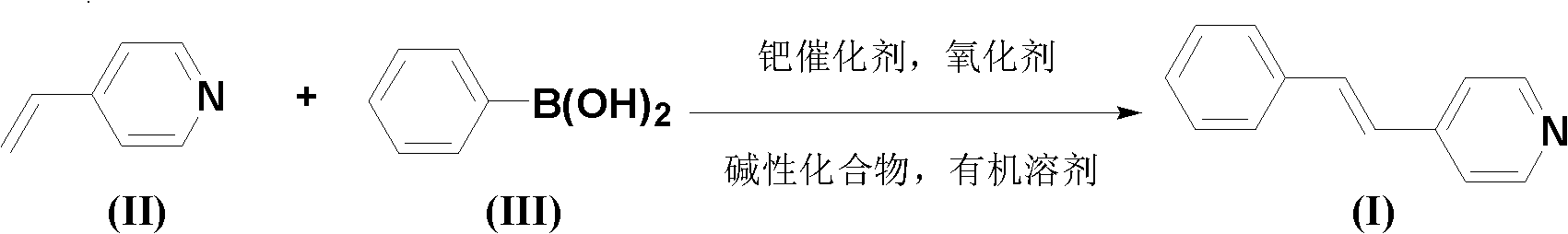
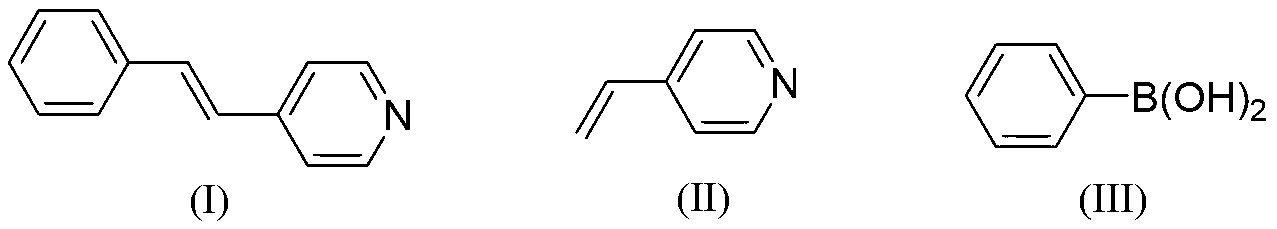
Chemical synthesis method for (E)-4-styryl pyridine
A technology of styrylpyridine and vinylpyridine, which is applied in the field of chemical synthesis of -4-styrylpyridine, can solve the problems of limited promotion, harsh reaction conditions, low selectivity and yield of E-configuration products, and achieve The effect of advanced and reasonable process route, mild reaction conditions, great implementation value and social and economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The amount ratio of feed material 4-vinylpyridine: phenylboronic acid: palladium catalyst: oxidizing agent: basic compound is 1.0: 1.0: 0.05: 0.05: 2.0 Feeding, 4-vinylpyridine 21.0g (0.2mol); Phenylboronic acid 24.4g (0.2mol); The palladium catalyst is di(acetylacetonate) palladium, and the feeding quality is 3.04g (0.01mol); the oxidizing agent is copper acetate monohydrate, and the feeding quality is 1.99g (0.01mol); the basic compound is sodium bicarbonate, and the feeding quality 33.6g (0.4mol); the organic solvent is 210g of N,N'-dimethylformamide, the total amount of which is 10 times the mass of 4-vinylpyridine.
[0027] Dissolve phenylboronic acid, palladium catalyst, oxidant and basic compound in an organic solvent (the amount of the organic solvent used is 6 times the mass of 4-vinylpyridine). Dissolve 4-vinylpyridine in an organic solvent (the amount of organic solvent is 4 times the mass of 4-vinylpyridine), slowly add dropwise to the above solution of phen...
Embodiment 2
[0032] The amount ratio of feed material 4-vinylpyridine: phenylboronic acid: palladium catalyst: oxidizing agent: basic compound is 1.0: 1.5: 0.05: 0.05: 2.0 Feeding, 4-vinylpyridine 21.0g (0.2mol); Phenylboronic acid 36.6g (0.3mol); The palladium catalyst is di(acetylacetonate) palladium, and the feeding quality is 3.04g (0.01mol); the oxidant is copper acetate monohydrate, and the feeding quality is 1.99g (0.01mol); the basic compound is sodium bicarbonate, and the feeding quality 33.6g (0.4mol); the organic solvent is 210g of N,N'-dimethylformamide, the total amount of which is 10 times the mass of 4-vinylpyridine.
[0033] The reaction temperature was 90°C, the reaction time was 24 hours, and other operations were the same as in Example 1. (E)-4-Styrylpyridine was 26.4g, the yield was 73%, and the purity was 98.5%.
Embodiment 3
[0035] The amount ratio of feed material 4-vinylpyridine: phenylboronic acid: palladium catalyst: oxidizing agent: basic compound is 1.0: 2.0: 0.05: 0.1: 3.0 feeding, 4-vinylpyridine 21.0g (0.2mol); Phenylboronic acid 48.8g (0.4mol); The palladium catalyst is di(acetylacetonate) palladium, and the feeding quality is 3.04g (0.01mol); the oxidant is copper acetate monohydrate, and the feeding quality is 3.98g (0.02mol); the basic compound is sodium bicarbonate, and the feeding quality 50.4g (0.6mol); the organic solvent is 210g of N,N'-dimethylformamide, the total amount of which is 10 times the mass of 4-vinylpyridine.
[0036] The reaction temperature is 90°C, the reaction time is 36 hours, other operations are the same as in Example 1, (E)-4-styrylpyridine is 27.5g, the yield is 76%, and the purity is 98.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com