Method for synthesizing 3-aryl sulfydryl indole compound
A technology of aryl mercapto indole and synthesis method, which is applied in the field of chemical synthesis of 3-aryl mercapto indole compounds, can solve problems such as application limitation, and achieve the effects of low cost, high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
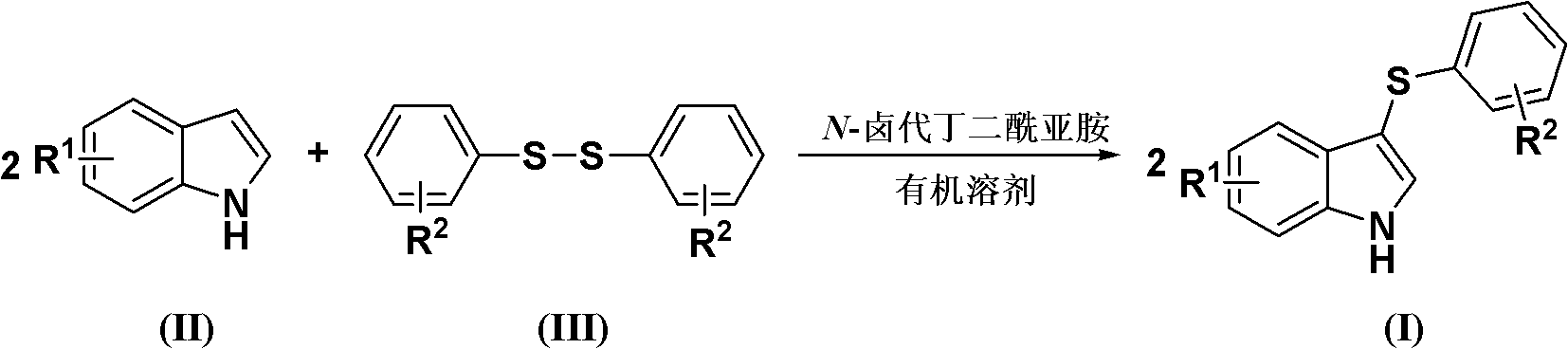
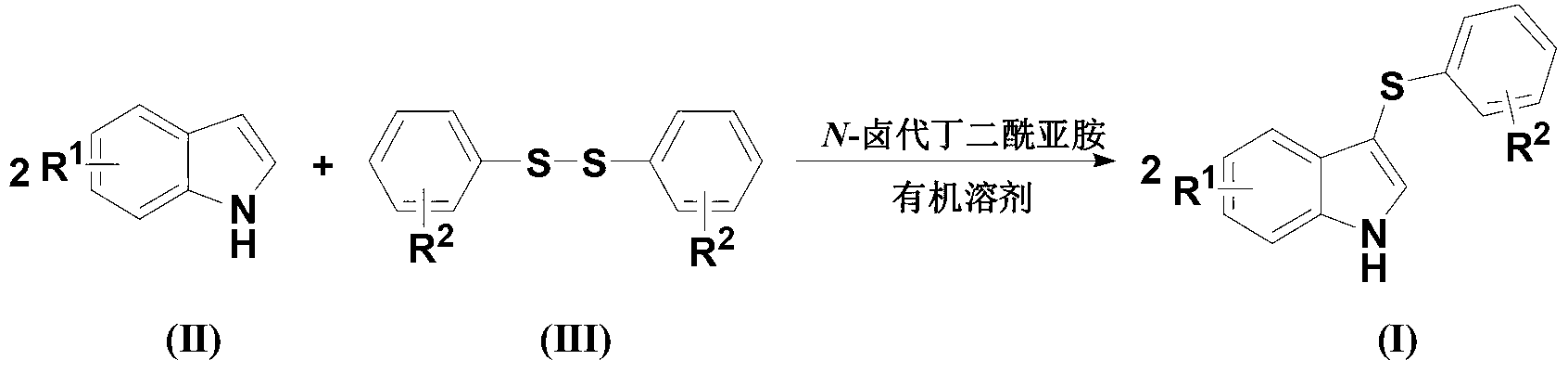
[0022] Diaryl disulfide, indole compound and N-halogenated succinimide are fed in a molar ratio of 1.0:2.0:2.5, and the diaryl disulfide is diphenyl disulfide, and the feeding quality is 21.8g (0.1mol); The indole compound is indole, and the feeding quality is 23.4g (0.2mol); N-halogenated succinimide is N-bromosuccinimide, and the feeding quality is 44.5g (0.25mol) ; The organic solvent is N,N,-218g of dimethylformamide, and its total consumption is 10 times of the quality of diaryl disulfide.
[0023] Dissolve indole and N-halogenated succinimide in an organic solvent (the amount of organic solvent used is 6 times the mass of diphenyl disulfide). Diaryl disulfide is dissolved in an organic solvent (the amount of organic solvent is 4 times the mass of diphenyl disulfide), slowly added dropwise to the solution of indole compounds and N-halogenated succinimide, The reaction temperature was -15°C, and the reaction was completed after 3 hours.
[0024] After completion of the r...
Embodiment 2
[0027] Diaryl disulfide, indole compound and N-halogenated succinimide are fed in a molar ratio of 1.0:2.0:2.5, and the diaryl disulfide is diphenyl disulfide, and the feeding quality is 21.8g (0.1mol); The indole compound is indole, and the feeding quality is 23.4g (0.2mol); N-halogenated succinimide is N-chlorosuccinimide, and the feeding quality is 33.4g (0.25mol) ; The organic solvent is N,N,-218g of dimethylformamide, and its total consumption is 10 times of the quality of diaryl disulfide.
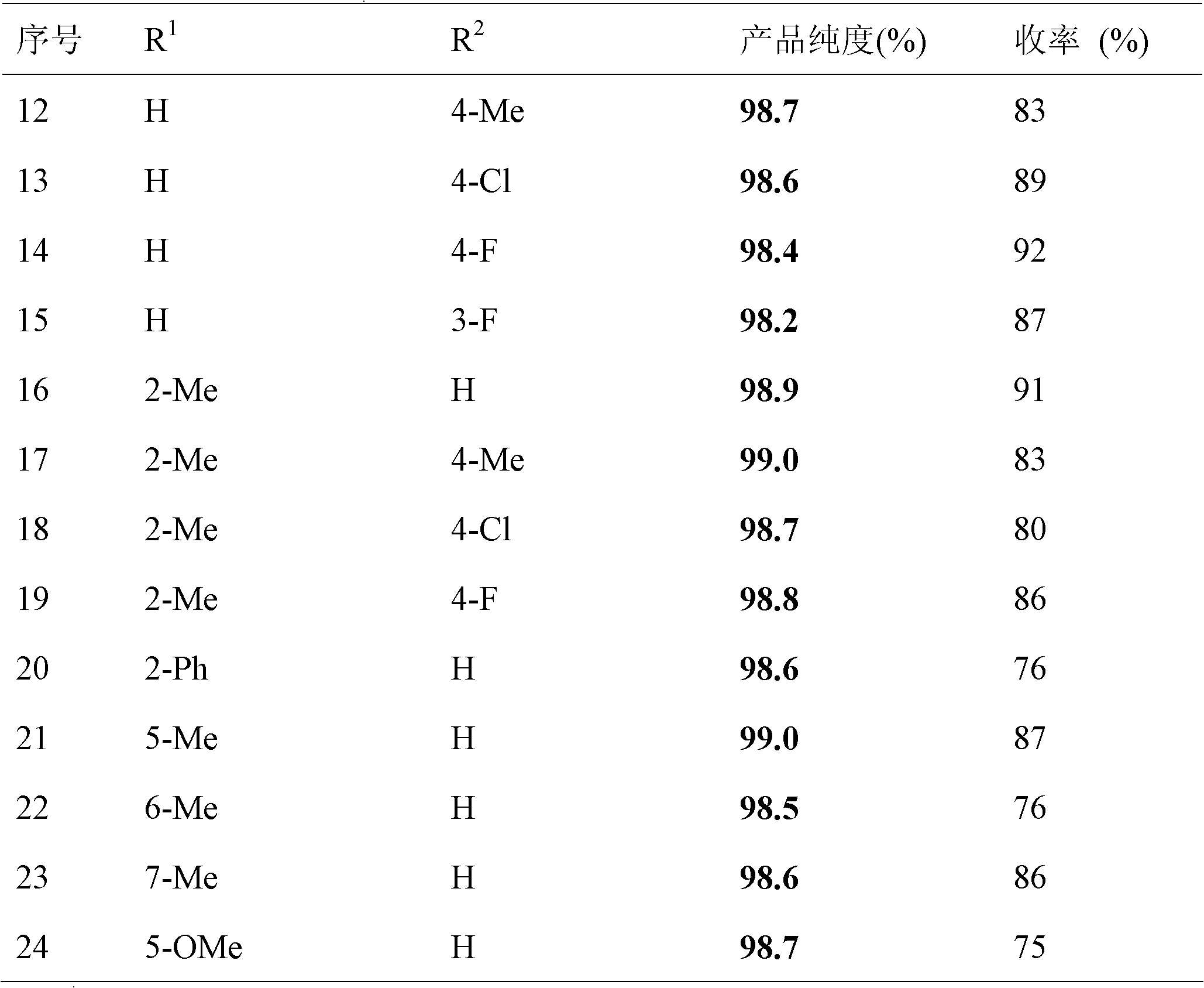
[0028] The rest are the same as in Example 1, the resulting product 3-phenylmercaptoindole is 22.1g, the yield is 49%, and the purity is 98.8%.
Embodiment 3
[0030] Diaryl disulfide, indole compound and N-halogenated succinimide are fed in a molar ratio of 1.0:2.0:2.5, and the diaryl disulfide is diphenyl disulfide, and the feeding quality is 21.8g (0.1mol); The indole compound is indole, and the feeding quality is 23.4g (0.2mol); N-halogenated succinimide is N-iodosuccinimide, and the feeding quality is 56.3g (0.25mol) ; The organic solvent is N,N,-218g of dimethylformamide, and its total consumption is 10 times of the quality of diaryl disulfide.
[0031] The rest are the same as in Example 1, the resulting product 3-phenylmercaptoindole is 23.9g, the yield is 53%, and the purity is 98.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com