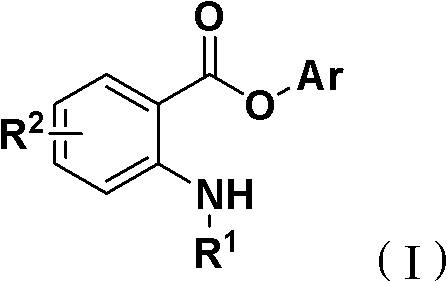
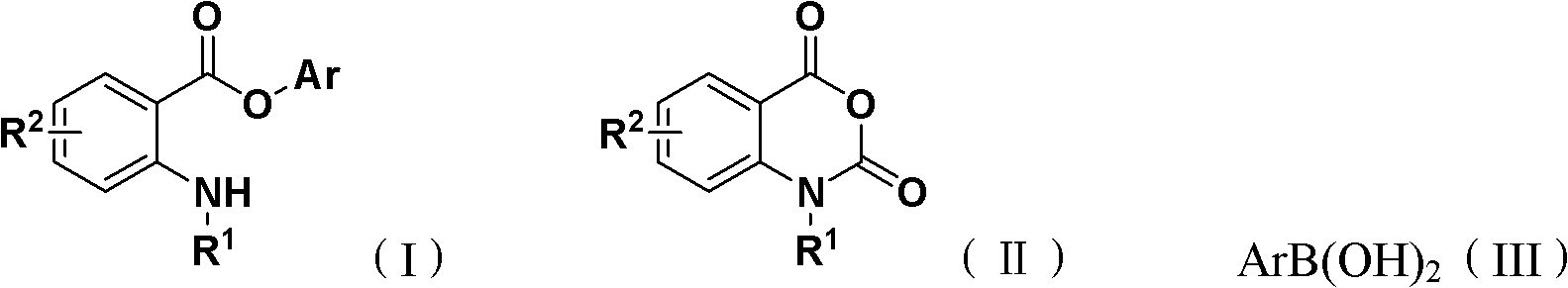
Synthesis method for ortho amino aromatic formic acid aryl ester derivatives
A technology of amino arylcarboxylic acid and synthesis method, which is applied in the field of chemical synthesis of aryl ortho-amino aryl carboxylic acid derivatives, can solve problems such as poor functional group compatibility, low reaction selectivity, and limitations in promotion, and achieves great practical value. and social and economic benefits, less catalyst dosage, advanced and reasonable process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Isatoic anhydride, phenylboronic acid, tris(dibenzylideneacetone) dipalladium, bis(2-diphenylphosphine) phenyl ether, and 1-methylpiperidine are 1.0: 3.0: 0.05: 0.1: 3.0 feed intake, isatoic anhydride 16.3g (0.1mol); phenylboronic acid 36.6g (0.3mol); tris (dibenzylidene acetone) dipalladium 4.58g (0.005mol); bis (2-diphenylphosphine) phenyl ether 5.39g (0.01mol); 29.7g (0.3mol) of 1-methylpiperidine; the organic solvent is tetrahydrofuran 195.6g, and its total amount is 10 times the mass of isatoic anhydride.
[0026] Put isatoic anhydride, phenylboronic acid, tris(dibenzylideneacetone) dipalladium, bis(2-diphenylphosphine) phenyl ether, and 1-methylpiperidine into the reaction kettle, add tetrahydrofuran to dissolve, and the reaction temperature is 60 °C, the reaction was complete after 24 hours.
[0027] After the reaction is over, the organic solvent is reclaimed by distillation under reduced pressure, and the solid remaining in the reaction flask is recrystallized...
Embodiment 2
[0030] Isatoic anhydride, phenylboronic acid, tris(dibenzylideneacetone) dipalladium, tricyclohexylphosphine, triethylamine are 1.0: 3.0: 0.05: 0.1: 3.0 in material ratio, and isatoic anhydride 16.3g (0.1 mol); 36.6g (0.3mol) of phenylboronic acid; 4.58g (0.005mol) of three (dibenzylideneacetone) dipalladium; 2.8g (0.01mol) of tricyclohexylphosphine; 30.4g (0.3mol) of triethylamine; The organic solvent is tetrahydrofuran 163g, and its total consumption is 10 times of the mass of isatoic anhydride.
[0031] All the other are the same as in Example 1, the resulting product phenyl anthranilate 17.7g, yield 83%, purity 98.5%.
Embodiment 3
[0033] Isatoic anhydride, phenylboronic acid, tris(dibenzylideneacetone) dipalladium, bis(2-diphenylphosphine) phenyl ether, and 1-methylpiperidine are 1.0: 3.0: 0.05: 0.1: 3.0 feed intake, isatoic anhydride 16.3g (0.1mol); phenylboronic acid 36.6g (0.3mol); tris (dibenzylidene acetone) dipalladium 4.58g (0.005mol); bis (2-diphenylphosphine) phenyl ether 5.39g (0.01mol); 29.7g (0.3mol) of 1-methylpiperidine; 163g of 1,4-dioxane as the organic solvent, and its total amount is 10 times the mass of isatoic anhydride.
[0034] All the other are the same as in Example 1, the resulting product phenyl anthranilate 14.7g, yield 69%, purity 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com