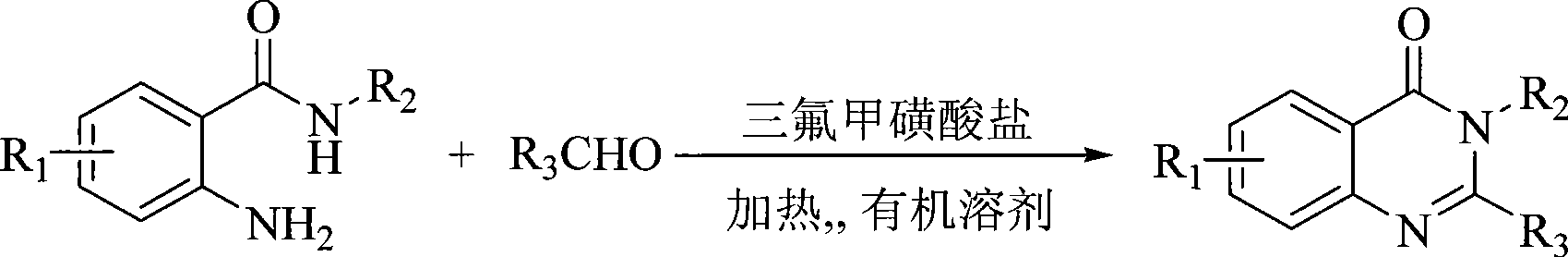
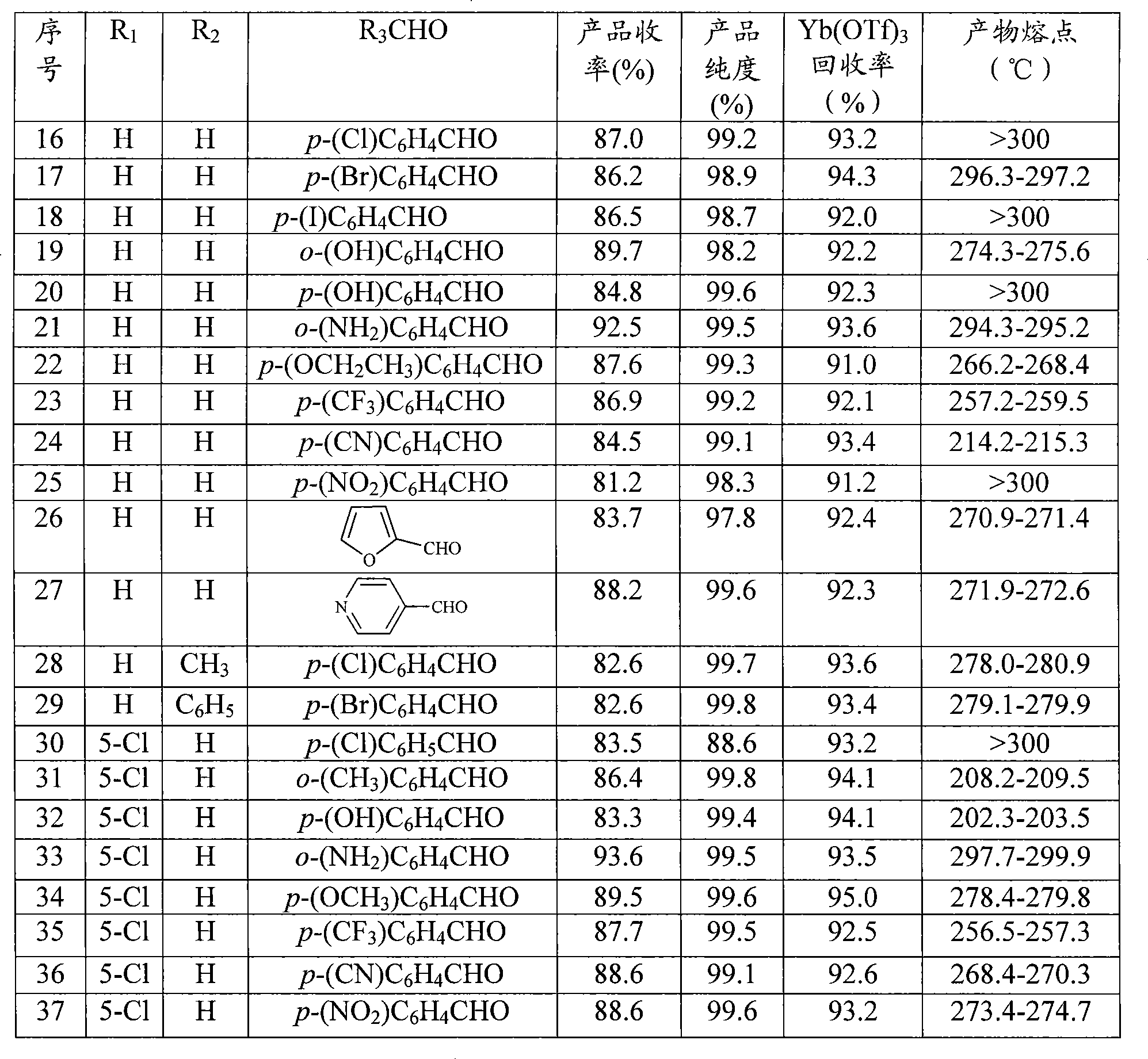
Synthesis of quinazoline ketone compounds
A quinazolinone and synthesis method technology, applied in the field of green chemical synthesis of quinazolinone compounds, can solve problems such as unrecyclable, serious environmental pollution, excessive catalyst, etc. The route is advanced and reasonable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The ratio of the feed materials of o-amino-substituted benzamide, aldehyde, and zinc trifluoromethanesulfonate is 1:1:0.05, the o-amino-substituted benzamide is anthranilamide, and the aldehyde is benzaldehyde.
[0026] In a 150ml three-neck flask equipped with a thermometer, a reflux condenser and mechanical stirring, add 13.6g (0.1mol) of anthranilamide, 10.6g (0.1mol) of benzaldehyde, and 1.8g (5mmol) of zinc trifluoromethanesulfonate into nitroethane (30 mL), heated to 105° C., and reacted for 40 minutes. At the same time, HPLC was used to track and monitor (flow rate: 1.5ml / min, acetonitrile: water: acetic acid = 40:60:0.1). After the reaction was completed, cool to room temperature, add 20mL of water, and keep stirring for 10 minutes, the solid precipitated, and the insoluble matter was filtered out to obtain a crude product, which was recrystallized with ethanol to obtain a white solid of 2-phenyl-4(3H)-quinazoline Ketone 19.7g, yield 89%, purity 99.3%, melting ...
Embodiment 2
[0029] The ratio of the feed materials of o-amino-substituted benzamide, aldehyde, and scandium trifluoromethanesulfonate is 1:1:0.05, the o-amino-substituted benzamide is anthranilamide, and the aldehyde is benzaldehyde. 13.6 g (0.1 mol) of anthranilamide, 10.6 g (0.1 mol) of benzaldehyde, and 2.46 g (5 mmol) of scandium trifluoromethanesulfonate.
[0030] Others were the same as in Example 1 to obtain 20.2 g of the product 2-phenyl-4(3H)-quinazolinone, with a yield of 91%, a purity of 99.5%, a melting point of 238° C., and a recovery rate of scandium trifluoromethanesulfonate of 92.5%.
Embodiment 3
[0032] The ratio of the feed materials of o-amino-substituted benzamide, aldehyde, and lanthanum trifluoromethanesulfonate is 1:1:0.05, the amino-substituted benzamide is anthranilamide, and the aldehyde is benzaldehyde. 13.6 g (0.1 mol) of anthranilamide, 10.6 g (0.1 mol) of benzaldehyde, and 2.9 g (5 mmol) of lanthanum trifluoromethanesulfonate.
[0033] Others are the same as in Example 1 to obtain 20.4 g of the product 2-phenyl-4(3H)-quinazolinone, with a yield of 92%, a purity of 97.7%, a melting point of 237~238°C, and a recovery rate of lanthanum trifluoromethanesulfonate of 93.9% .
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com