Anti infectious compound and usage
A compound and anti-infection technology, which is applied in the field of quinolone anti-infection drugs, can solve problems affecting the use of drugs, and achieve the effect of adding new varieties, definite structure, and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
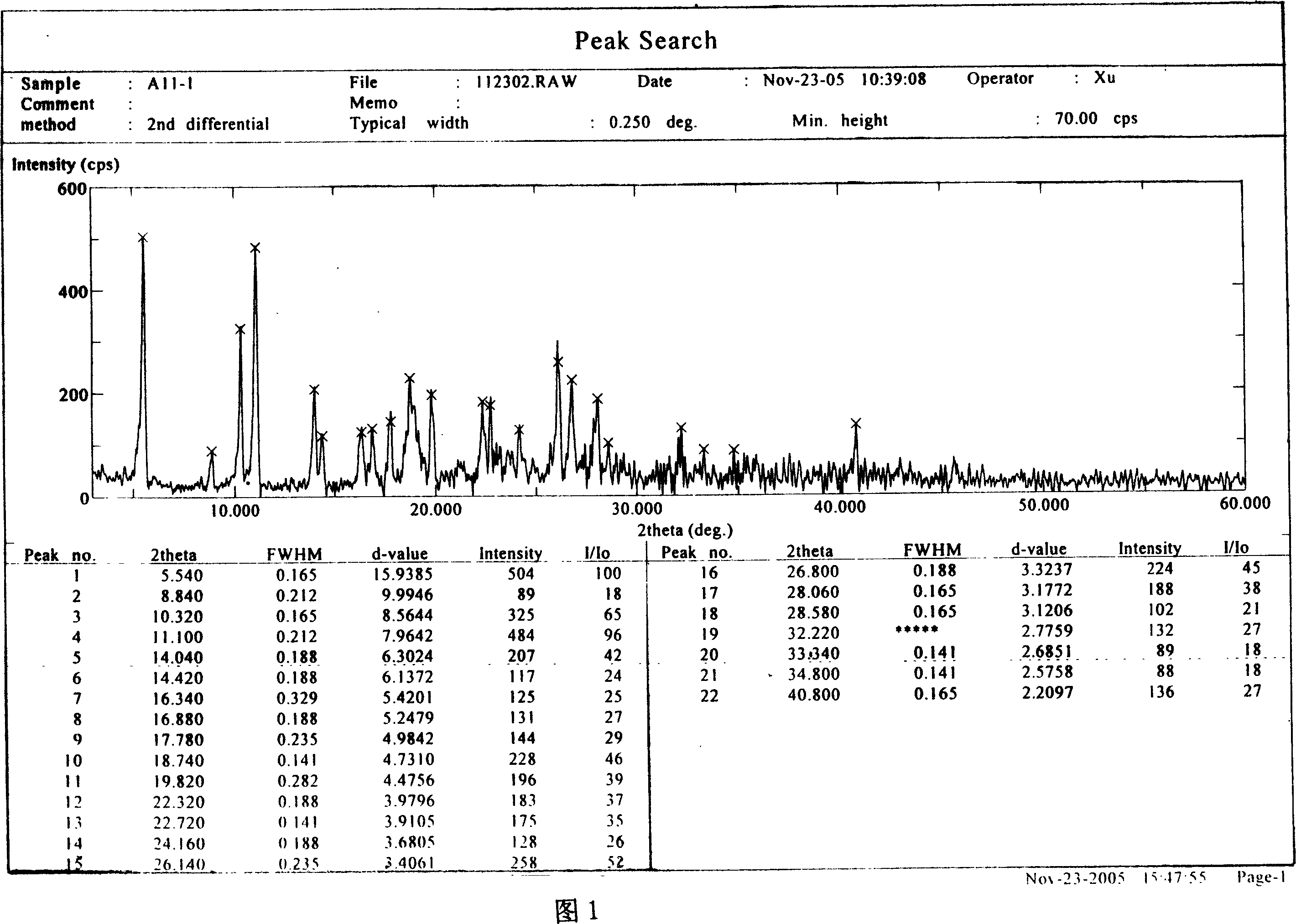
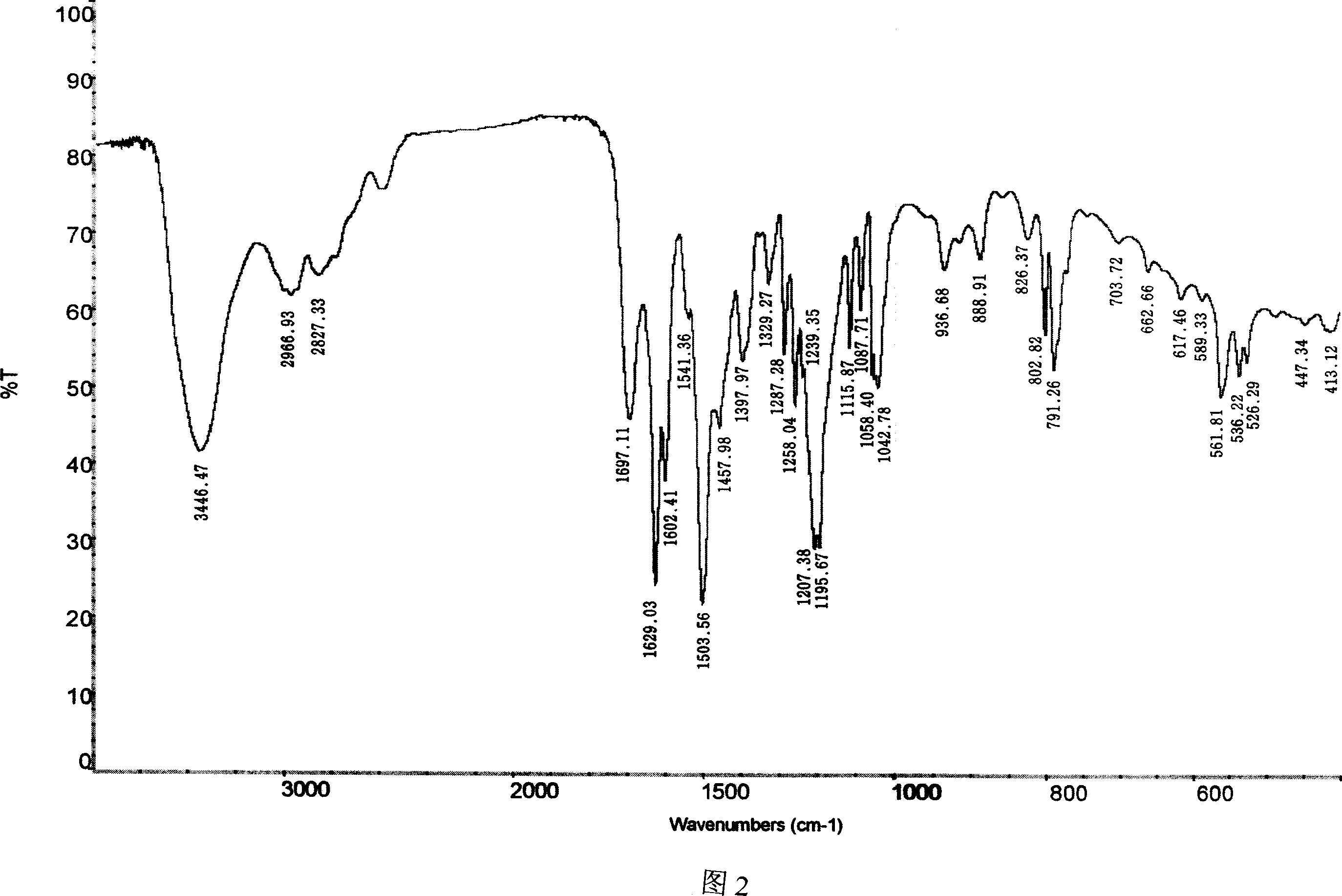
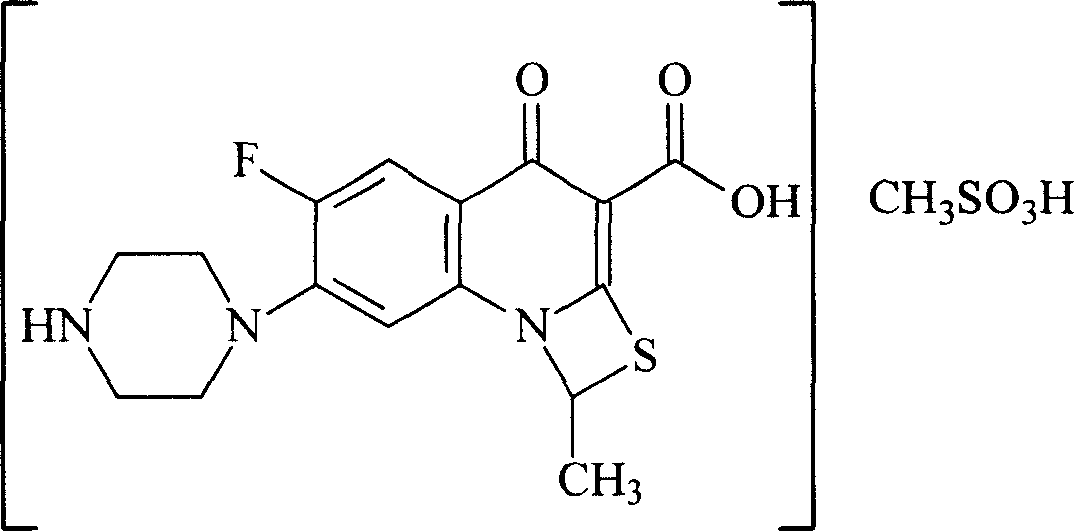
Image
Examples
Embodiment 1
[0086] Add 50L of water and 5L of ethanol into a 100L reaction tank, add 10Kg of compound II at 15-20°C, add 2.8Kg of methanesulfonic acid dropwise under stirring, stir for 30 minutes, add 0.5Kg of activated carbon for needles, stir for 15 minutes, and filter to 500L crystallization tank; wash the reaction tank with 10L water and filter into the 500L crystallization tank. Under stirring, about 350 L of isopropanol was added dropwise into the crystallization tank, and the addition of isopropanol was completed in 3 hours, and a solid was precipitated. Keep warm at 5-10°C and stir for 2 hours, filter, wash once with 20 L of isopropanol, and vacuum-dry at 35-40°C. 8.7 Kg of compound I were obtained.
[0087] The product elemental analysis results are as follows:
[0088] Analysis Project
C
H
N
S
measured value%
52.11
4.86
9.56
7.39
52.21
4.79
9.64
7.28
Calculated...
Embodiment 2
[0114] Add 50L of water and 15L of acetone into the reaction tank, add 10Kg of compound II at 10-15°C, add 2.8Kg of methanesulfonic acid dropwise under stirring, stir for 1 hour, add 0.5Kg of activated carbon for needles, stir for 20 minutes, filter and decarburize ; The filtrate is pressed into a 500L crystallization tank in a sterile room through a 0.22μm ultrafiltration membrane. 10L of water washes the reaction tank and pipeline, and is also pressed into the crystallization tank. Stirring was started, and 350 L of acetone was added dropwise. The addition of acetone was completed in 4 hours, and a solid precipitated. Keep warm at 0-5°C and stir for 5 hours, filter, wash twice with acetone 30L×2, and vacuum-dry at 35-40°C. Obtain 8.4Kg of sterile powder of Compound I.
Embodiment 3
[0116] Add 50ml of water into a 100ml three-neck flask, add 10g of compound II at 0-5°C, add 4.1g of methanesulfonic acid dropwise under stirring, stir for 30 minutes, add 0.5g of activated carbon for needles, stir for 15 minutes, filter and decarburize ; Wash the three-necked bottle with 10ml of water and filter. Combine the filtrates into a 500ml three-neck flask, add dropwise 350ml of ethanol under stirring, and finish adding the mixed solution in 2 hours, and a solid precipitates out. Keep warm at 15-20°C and stir for 2 hours, filter, wash once with 20ml of absolute ethanol, and vacuum-dry at 35-40°C. 6.9 g of compound I are obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com