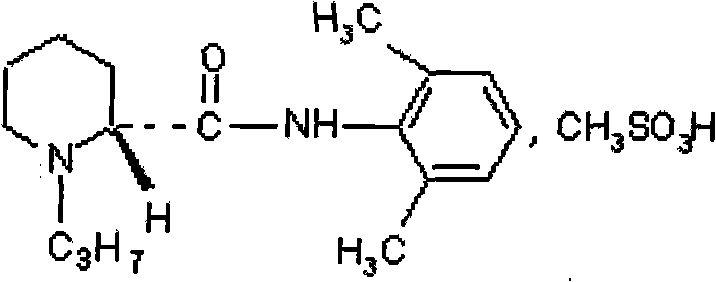
Preparation method of ropivacaine mesylate injection packed by soda-lime glass bottle
A technology of ropivacaine mesylate and vials, which is applied in the field of medical injections, and can solve the problem of poor chemical stability and thermal stability of neutral glass, poor ability to withstand temperature changes, hand scratches of medical staff, ampoule packaging Bottles are crushed and other problems, to achieve the effect of convenient clinical application, avoid pollution, and reduce the probability of occurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Take 100 ropivacaine mesylate injections of production vial packaging as an example and its preparation method is as follows:
[0041] 1. Ingredients
[0042] Prepare the ropivacaine mesylate injection 1000mL of vial packaging according to the following ingredients:
[0043] Ropivacaine Mesylate 8940mg
[0045] Add water for injection to 1000mL
[0046] 2. Dosing
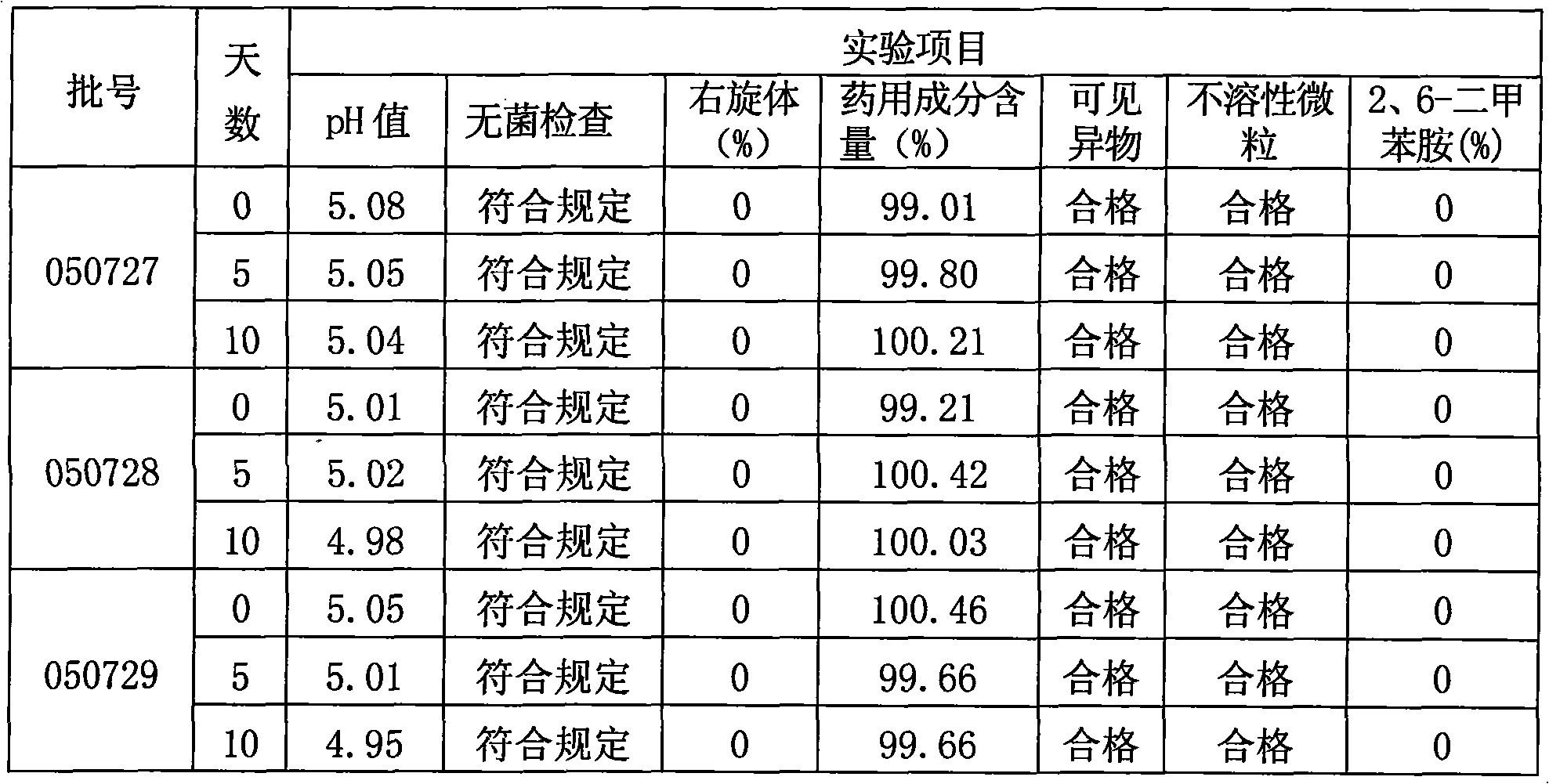
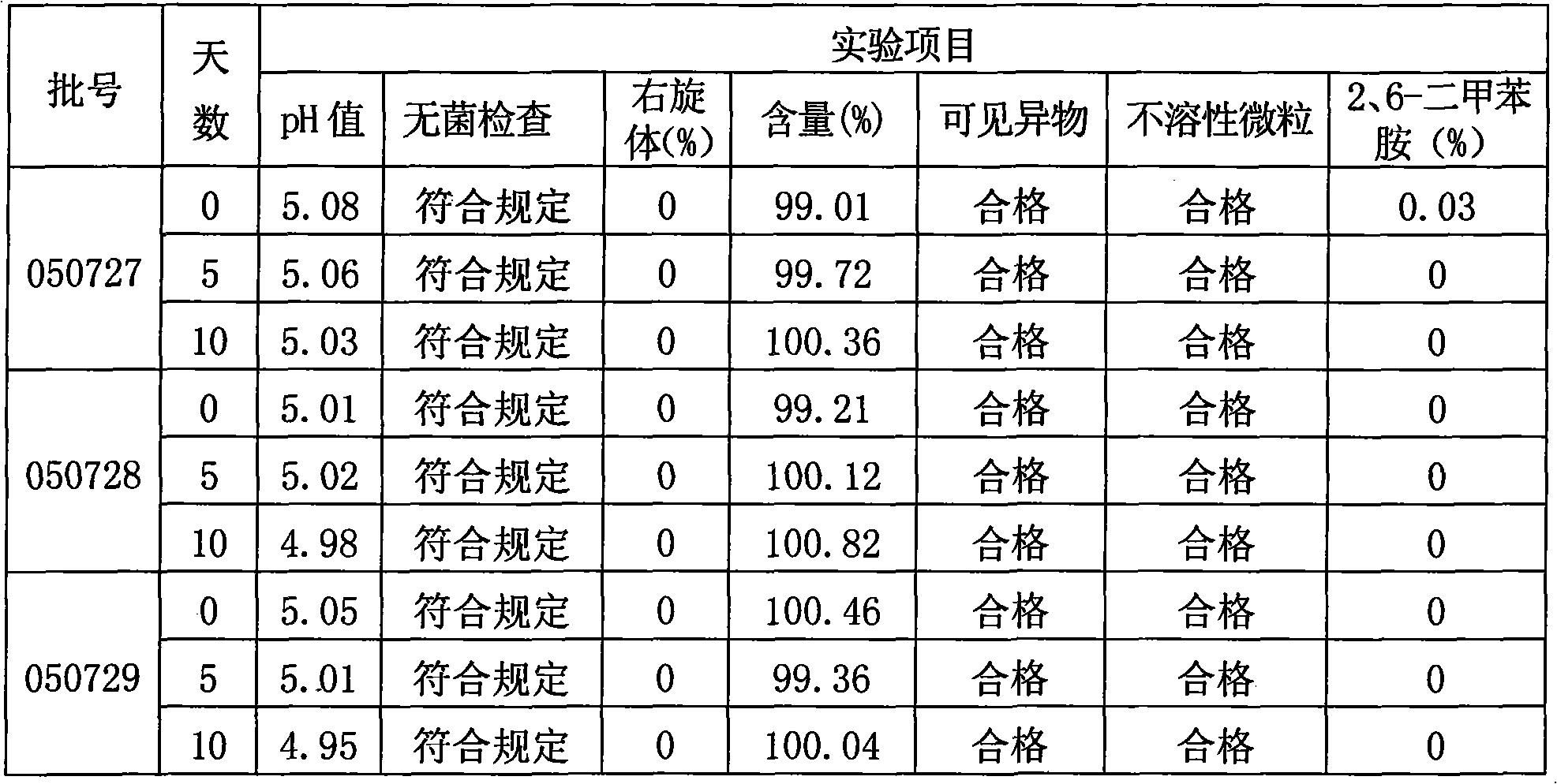
[0047] Take 800ml of water for injection in the JCG-150 liquid mixing tank, fill it with nitrogen, the nitrogen flow rate is 10L / min, add sodium chloride and ropivacaine mesylate, stir to dissolve it fully, and oxidize it with 0.1moL / L hydroxide Sodium solution to adjust the pH to 4.25±0.1; add 0.02% by weight of activated carbon with a concentration of g / ml to the solution, stir at 40-50°C for 15 minutes, filter and decarburize the titanium rod, and take samples to detect the pH value of the intermediate product and methanesulfonic acid For the content of ropivacaine, add...
Embodiment 2
[0065] Take 100 ropivacaine mesylate injections of production vial packaging as an example and its preparation method is as follows:
[0066] Ingredients:
[0067] Prepare the ropivacaine mesylate injection 1000mL of vial packaging according to the following ingredients:
[0068] Ropivacaine Mesylate 11920mg
[0070] Add water for injection to 1000mL
[0071] Its preparation method is as follows:
[0072] In the process step 3 of cleaning vials and rubber stoppers, the vials are ultrasonically cleaned, rinsed with circulating water, blown with clean compressed air, rinsed with injection water, dried with clean compressed air, and then optimized with a sterilizing dryer. Sterilize with dry heat at 260°C for 25 minutes; pour the rubber stopper into the KJCS-3 rubber stopper cleaning machine, wash it with water for injection, rinse with ion, and wash it with water for injection in sequence, and sterilize it with steam at 110°C for 30 minutes, h...
Embodiment 3
[0074] Take 50 ropivacaine mesylate injections of production vial packaging as an example and its preparation method is as follows:
[0075] Ingredients:
[0076] Prepare the ropivacaine mesylate injection 1000mL of vial packaging according to the following ingredients:
[0077] Ropivacaine Mesylate 2385mg
[0078] Sodium chloride 8600mg
[0079] Add water for injection to 1000mL
[0080] Its production method is as follows:
[0081] In the process step 3 of cleaning vials and rubber stoppers, the vials are ultrasonically cleaned, rinsed with circulating water, blown with clean compressed air, rinsed with injection water, dried with clean compressed air, and then optimized with a sterilizing dryer. Sterilize by dry heat at 410°C for 10 minutes; pour the rubber stopper into the KJCS-3 rubber stopper cleaning machine, wash it with water for injection, rinse with ion, and wash it with water for injection in sequence, then sterilize it with steam at 125°C for 30 minutes, and h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com