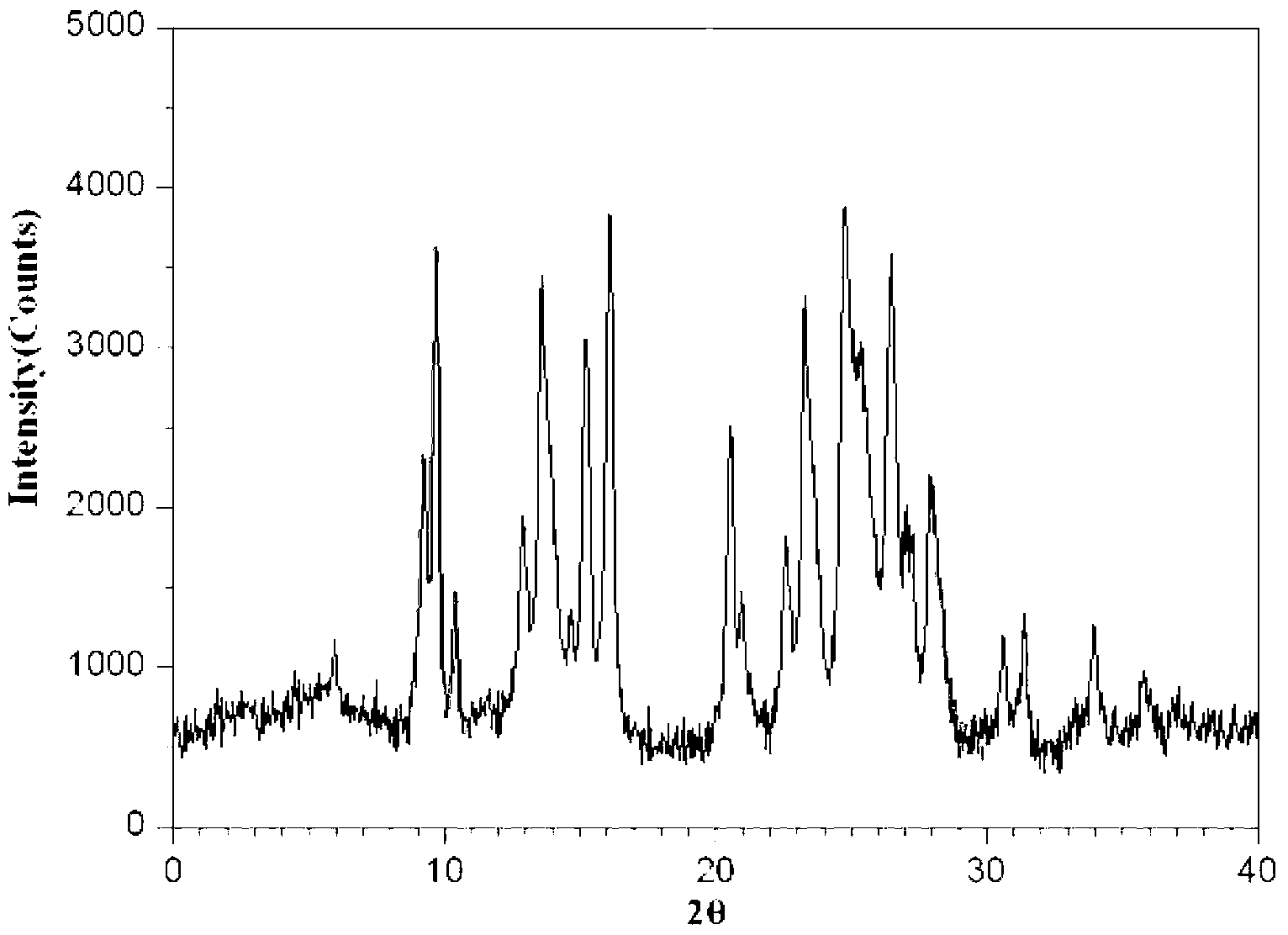
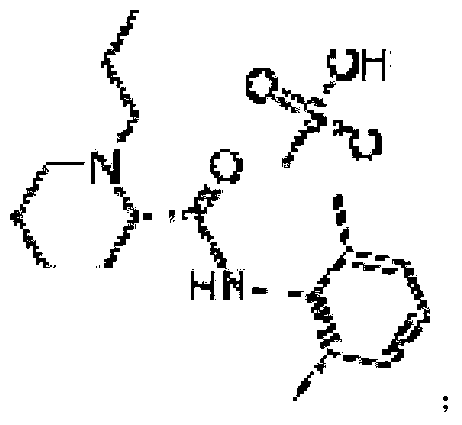
Ropivacaine mesylate compound, preparation process thereof and pharmaceutical composition thereof
A technology of ropivacaine mesylate and a compound, applied in the field of medicine, can solve the problems of slow dissolution rate, poor water solubility, low solubility and the like, and achieve the effects of high solubility, high solubility and easy compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation of ropivacaine mesylate compound:
[0044] Get ropivacaine mesylate crude drug 50g, add the mixed solution of DMF / isopropanol, wherein the volume ratio of DMF and isopropanol is 2:1, the consumption of ropivacaine mesylate crude drug and mixed solution The ratio is 1g: 15ml, heated to 45°C, after the ropivacaine mesylate crude drug is dissolved, add activated carbon for decolorization, the amount of activated carbon added is 0.2% g / ml of the total volume of the liquid, stir and adsorb for 25min, filter Decarbonize and sterilize, move the filtrate to a reaction kettle, put it in an oven at 150°C and let it stand for 48 hours, then lower the temperature to 55°C, open the kettle, adjust the pH to 9, and slowly add acetone under stirring conditions, the volume of acetone and the mixed solution The ratio was 3:1, the temperature was lowered to 5°C, filtered, washed 3 times with a mixed solution of DMF / isopropanol, and dried under reduced pressure for 5 hours ...
Embodiment 2
[0047] The preparation of ropivacaine mesylate compound:
[0048] Get ropivacaine mesylate crude drug 50g, add the mixed solution of DMF / isopropanol, wherein the volume ratio of DMF and isopropanol is 4:1, the consumption of ropivacaine mesylate crude drug and mixed solution The ratio is 1g:10ml, heated to 40°C, after the ropivacaine mesylate crude drug is dissolved, add activated carbon for decolorization, the amount of activated carbon added is 0.3% g / ml of the total volume of the liquid, stir and adsorb for 30min, filter Decarbonize and sterilize, move the filtrate to a reaction kettle, put it in an oven at 120°C and let it stand for 24 hours, then lower the temperature to 45°C, open the kettle, adjust the pH to 7, and slowly add acetone under stirring conditions, the volume of acetone and the mixed solution The ratio was 6:1, cooled to 0°C, filtered, washed 3 times with a mixed solution of DMF / isopropanol, and dried under reduced pressure for 4 hours to obtain a white powd...
Embodiment 3
[0051] Preparation of Ropivacaine Mesylate Small Volume Injection
[0052]
[0053] Take 70% of the prepared total amount of fresh water for injection at 70°C in the treated batching tank, and pass nitrogen for 60 minutes. Drop into the ropivacaine mesylate and sodium chloride prepared by the embodiment 1 of the prescription amount, stir and dissolve under nitrogen protection, add water for injection to the full amount, stir and mix evenly, adjust the pH to 5.5. Lower the temperature of the liquid medicine to 30°C, and continue nitrogen protection. Add 0.05% (g / ml) medicinal charcoal to the medicinal liquid, stir and adsorb for 20 minutes under nitrogen protection, and decarbonize and filter. Fine filter with a microporous membrane with a pore size of 0.45 μm, take a sample for inspection of visible foreign matter, and transport it to potting after meeting the requirements. Nitrogen filling and sealing: according to the specifications, it is filled and sealed in ampoules...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com