Pradefovir crystal
A technology of crystal and volume ratio, which is applied in the fields of pill delivery, digestive system, metabolic diseases, etc. It can solve the problems of unpublished compound crystallography, difficulty in obtaining single crystals, complex crystal distribution, etc., and achieve long-term storage, good temperature and moderate stability, high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
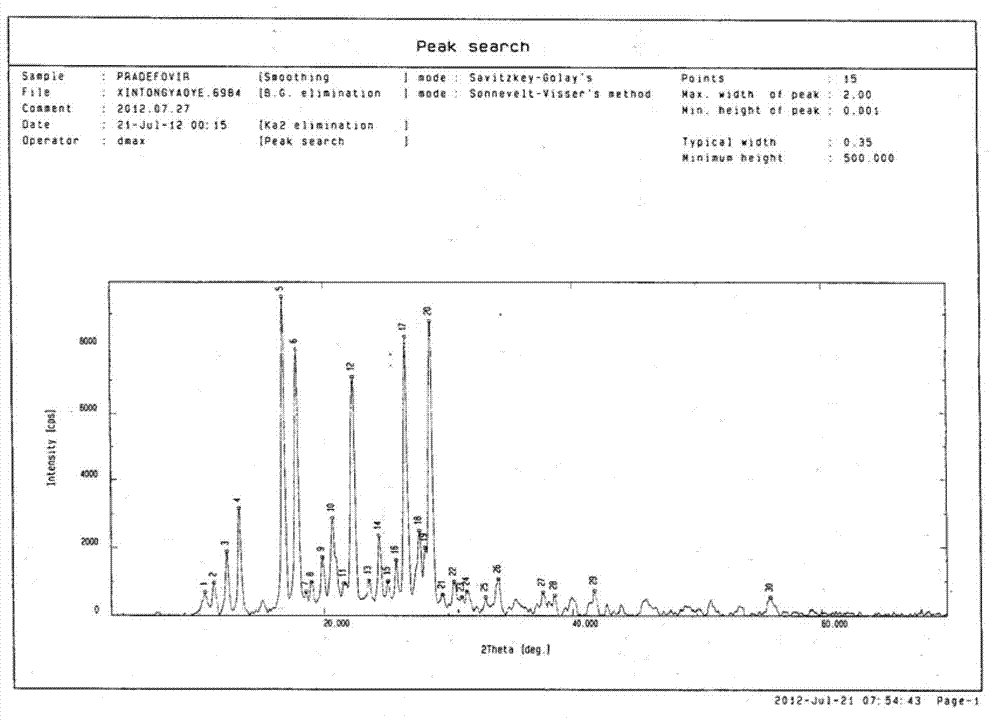
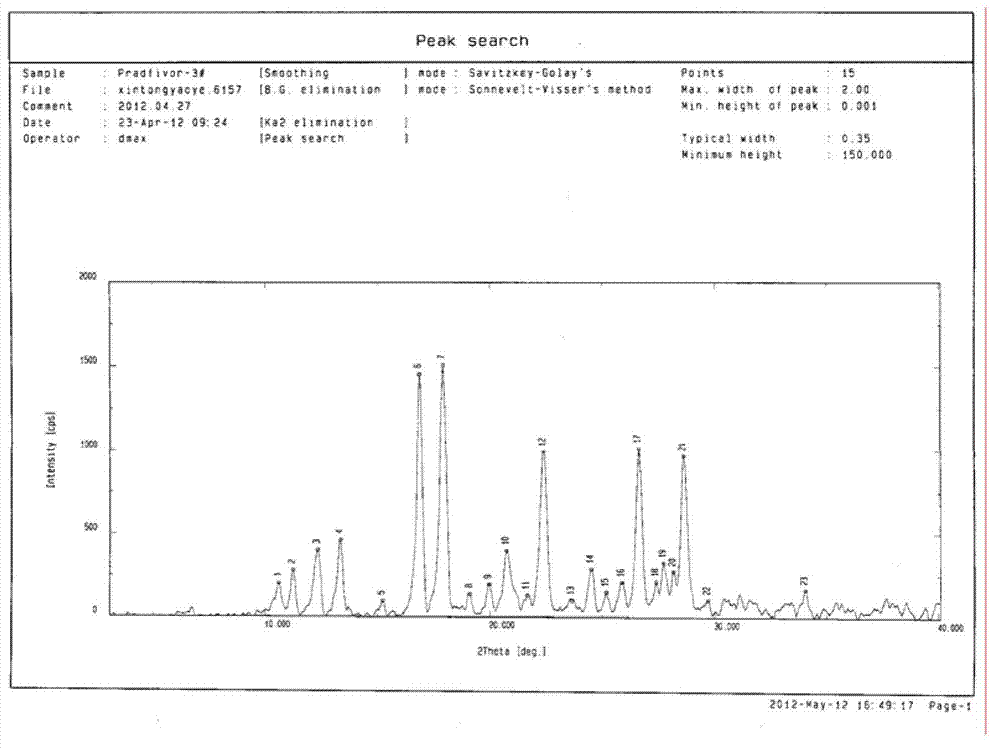
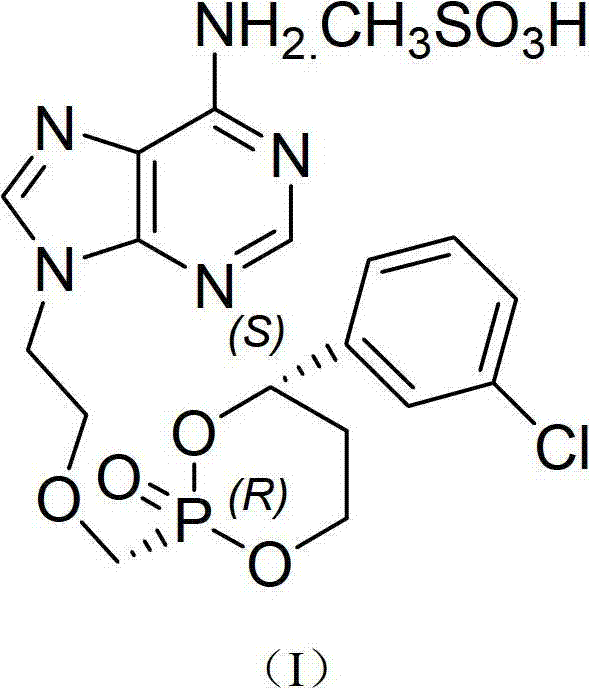
[0026] (+)-cis-9-{2-[4-[(S)-(3-Chlorophenyl)-2-oxo-1,3,2-dioxaphosphorin-2-methylene] Preparation and identification of type I crystal form of -1-ethyl}adenine mesylate
[0027] 1. Compound Synthesis
[0028] (+)-cis-9-{2-[4-[(S)-(3-chlorophenyl)-2-oxo-1,3,2- Dioxaphosphorane-2-methylene]-1-ethyl}adenine mesylate.
[0029] Specific steps are as follows:
[0030] step 1:
[0031]
[0032] Regent
[0033] Adefovir (1.25g, 4.58mmol) and N,N-methylformamide (0.5g, 4.95mmol) were added to 35ml of dichloromethane, and oxalyl chloride (1.4ml) was slowly added dropwise. The solvent was concentrated under reduced pressure to obtain a crude product, which was dissolved in 25 ml of dichloromethane, and the temperature was controlled at 0°C, and pyridine (0.75 mL, 9.16 mmol) was slowly added. Then the temperature was lowered to -78°C, and another part of dichloromethane (15mL) solution containing compound 5 (0.85g, 4.58mmol) and triethylamine (3.7mL, 29.06mmol) was added...
Embodiment 2
[0041] (+)-cis-9-{2-[4-[(S)-(3-Chlorophenyl)-2-oxo-1,3,2-dioxaphosphorin-2-methylene] Preparation and identification of the II crystal form of -1-ethyl}adenine mesylate
[0042] Add 1.2 g of the powder obtained in the above Example 1 "Synthesis of Compounds" into 60 ml of methanol containing 55% (V / V) water, stir while heating to 55 ° C until completely dissolved, then cool to 25 ° C, and find that The crystals were precipitated, filtered with suction to retain the crystals, and then directly dried in an oven at 55°C. The obtained crystal powder was tested with a PHI-5400 X-ray photoelectron analyzer (purchased from PE Company in the United States), and the test parameters were: voltage: 46kv, current: 40mA, copper kα radiation, λ: Test results such as figure 2 As shown in Table 2, it shows that stable type II single crystal can be obtained by this method.
[0043] Table 2 Peak List of Type II Crystals
[0044] Peak No.
[0045] 15
Embodiment 3
[0047] Stability Comparison of Crystals of the Invention and Compounds of the Prior Art
[0048] This example describes the crystals of the present invention (Type I crystals prepared in Example 1 and Type II crystals prepared in Example 2, respectively numbered as sample 1 and sample 2) and according to the prior art (Chinese patent application 200510098771.X, The number is sample 3) The stability comparison experiment of the compound prepared.
[0049] The high-temperature stability test was carried out at 65° C., and the results are shown in the table below, which shows that the crystal of the present invention has more high-temperature stability than the prior art.
[0050]
[0051] The stability test was carried out at 40°C for 6 months, and the results are shown in the table below, which shows that the crystals of the present invention are more stable under long-term storage than those of the prior art.
[0052]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com