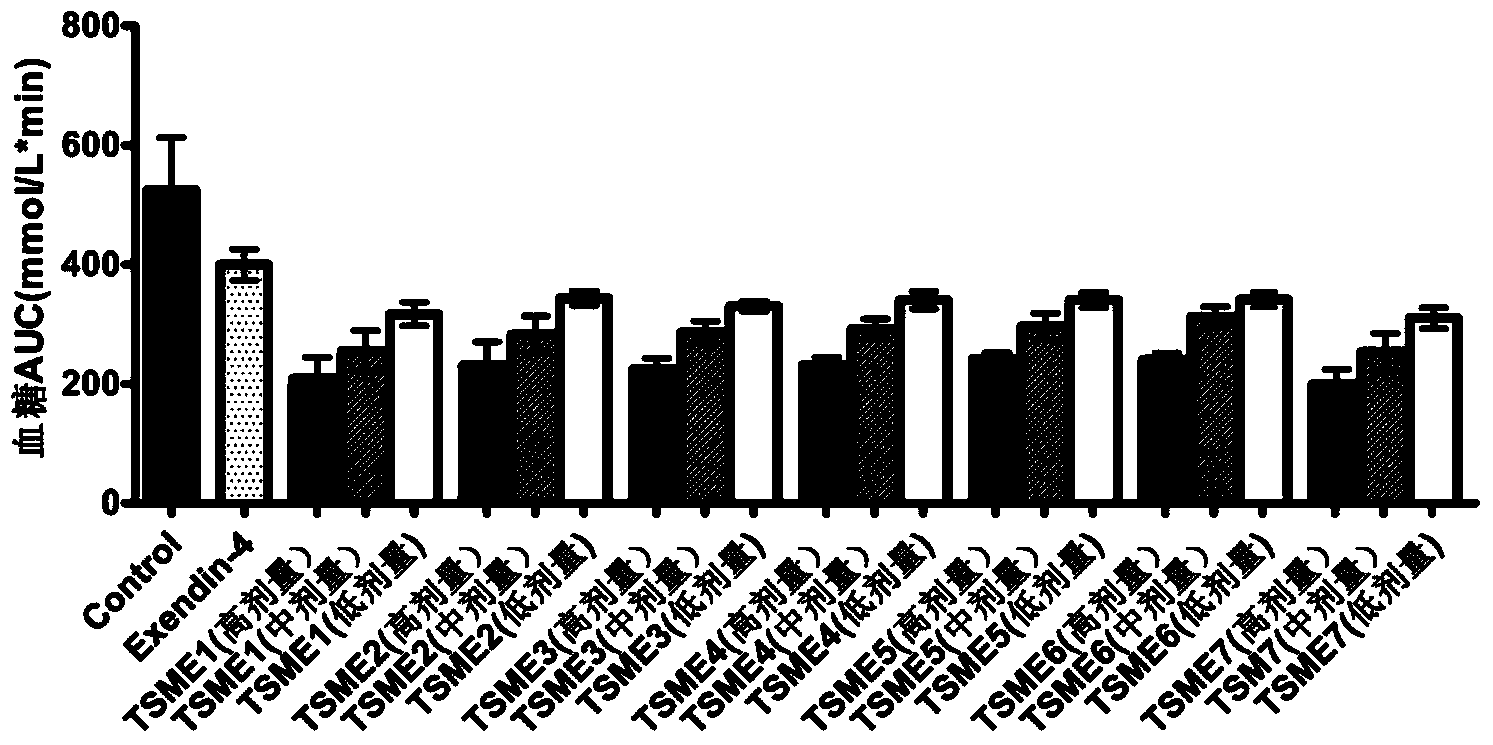Oral dosing hypoglycemic polypeptide as well as preparation method and application thereof
A technology for lowering blood sugar and medicine, applied in the field of medicine and biology, can solve problems such as difficult absorption, and achieve the effect of improving anti-enzymatic hydrolysis ability and good application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] TSME1-7 polypeptide oral hypoglycemic activity determination:
[0051] The oral hypoglycemic activity of TSME1-7 was evaluated by intraperitoneal glucose tolerance test (IPGTT):
[0052] Step 1: Prepare 1 mg / mL Exendin-4 solution and 1, 0.1, 0.01 mg / mL TSME1-7 solution with PBS solution. Then prepare a solution containing 25% PBS / Exendin-4 / TSME1-7, 25% propylene glycol and 50% NaHCO 3 solution (concentration of 3%) mixture.
[0053] Step 2: 138 male Balb / C mice were selected and randomly divided into 23 groups, 6 in each group. The groups and doses are as follows: blank control group (group 1) (PBS solution); positive control group (group 1) (Exendin-4 prototype molecule 500nmol / kg); TSME1-7 (group 3) in three doses (5nmol / kg). kg, 50nmol / kg, 500nmol / kg).
[0054] Step 3: Fasting the mice for 18 hours. Then blood was taken from the tail vein, and the blood glucose concentration was measured with a blood glucose meter as a 0-point blank blood sample.
[0055] The f...
Embodiment 2
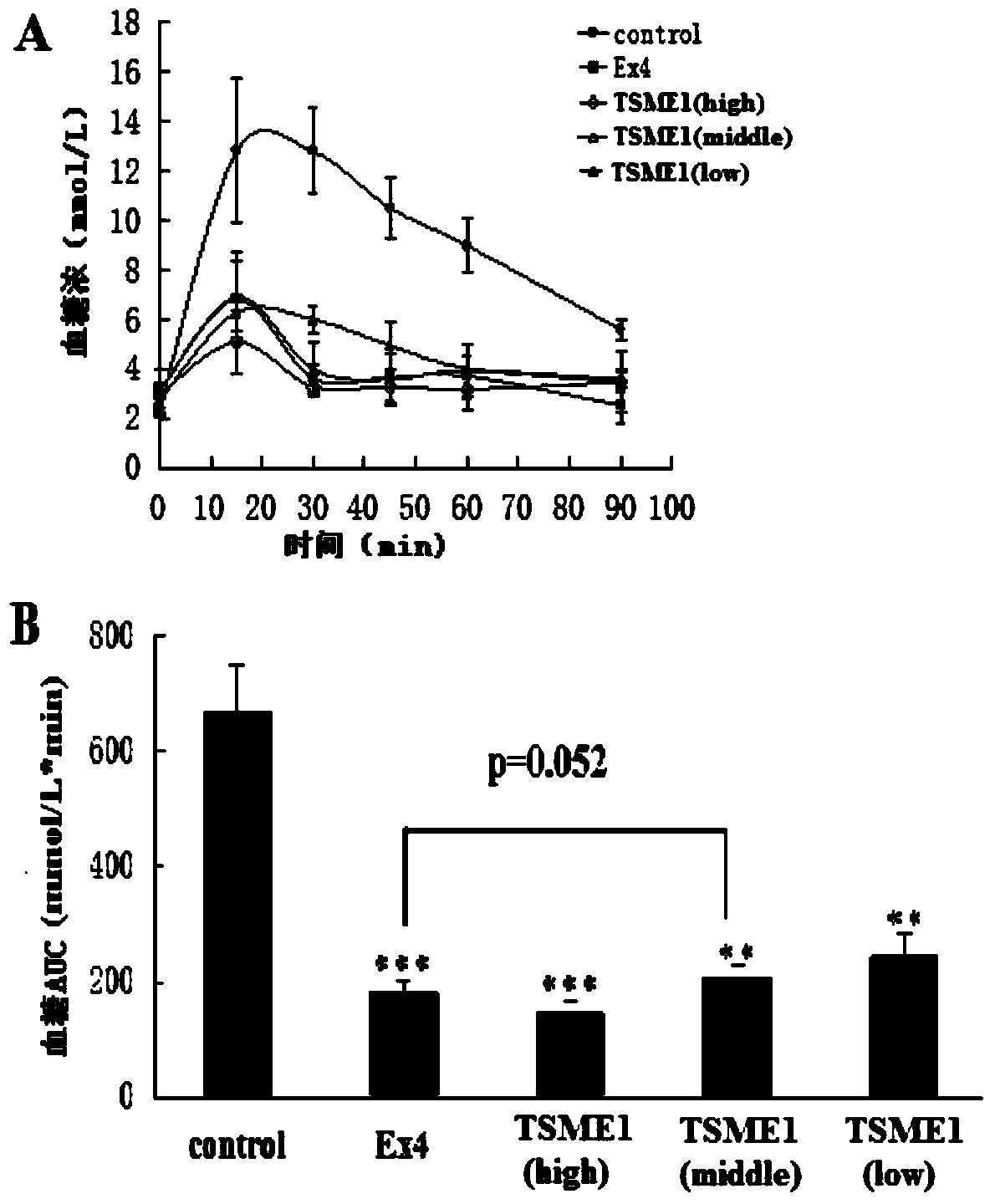
[0058] TSME1 biological activity detection in vivo:
[0059] The hypoglycemic activity of TSME was evaluated by intraperitoneal glucose tolerance test (IPGTT):
[0060] Step 1: Select 30 male Balb / C mice and randomly divide them into 5 groups with 6 mice in each group. The groups and doses are as follows: blank control group (group 1) (PBS solution (20mmol / L)); positive control group (group 1) (Exendin-4 prototype molecule 9nmol / kg); TSME1 (group 3) three doses (3nmol / kg, 9nmol / kg, 27nmol / kg).
[0061] Step 2: Fasting the mice for 18 hours. Then blood was taken from the tail vein, and the blood glucose concentration was measured with a blood glucose meter as a 0-point blank blood sample.
[0062] The third step: intraperitoneal injection to mice in each group (see the first step for the dosage).
[0063] The fourth step: intraperitoneally inject glucose solution 30 minutes after the administration (the dosage is the same as that in Example 1). Then blood was taken from th...
Embodiment 3
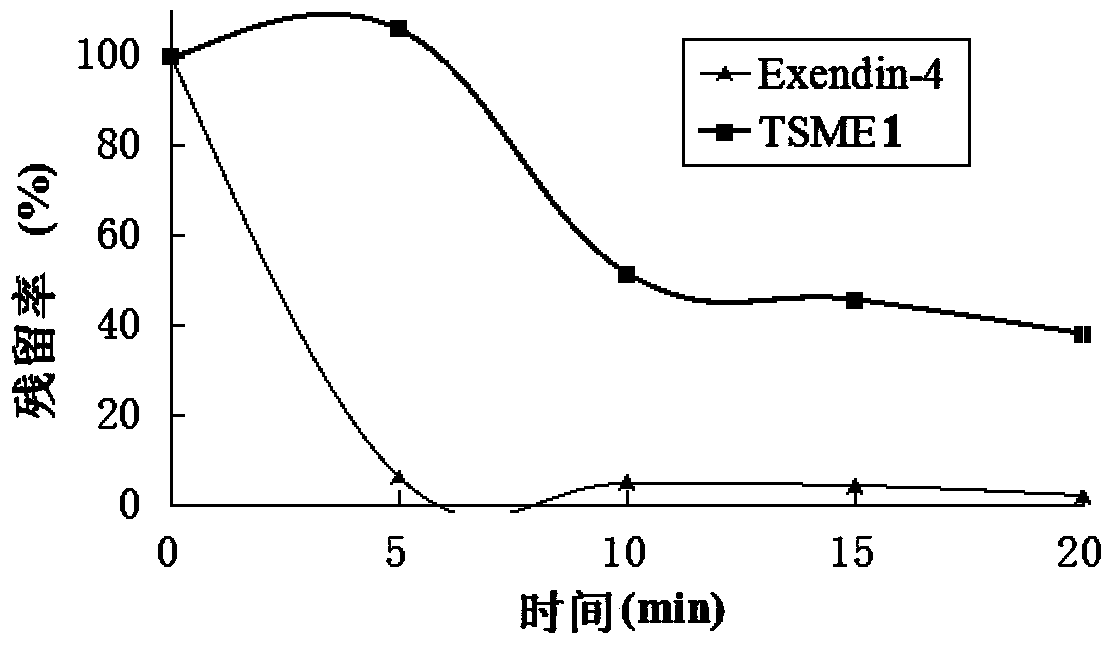
[0065] Determination of TSME1 resistance to enzymatic hydrolysis:
[0066] Step 1: Prepare 2mmol / L trypsin solution with PBS solution with pH=6.5. Incubate at 37°C for 30 minutes before use. At the same time, a 200ug / mL solution of Exendin-4 and TSME1 was prepared.
[0067] Step 2: Mix 20uL of Exendin-4 and TSME1 solution with 20uL of enzyme solution, and then stop the reaction with 100uL of 1% TFA solution at predetermined time points (0, 5, 10, 15, 20). After centrifugation at 12000rpm for 5min, the supernatant was detected by RP-HPLC.
[0068] Step 3: Calculate the residual rate. The residual rate of 0 point is 100%. The ratio of the peak area of each point to the peak area of 0 point is the residual rate of each point. see attached results image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com