Paeonol microemulsion preparation and preparation method thereof
A technology of paeonol and milk preparations, applied in the field of medicine, to achieve the effects of reducing the number of administrations, high biodegradability, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1. manual drop method prepares paeonol microemulsion preparation, and component and weight percentage thereof are as follows:
[0026] Paeonol 1%
[0027] Lecithin (surfactant) 6%
[0028] APG (surfactant) 12%
[0029] Propylene glycol (co-surfactant) 9%
[0030] Isopropyl myristate (oil phase) 12%
[0031] Deionized water 60%
[0032] The preparation method is as follows:
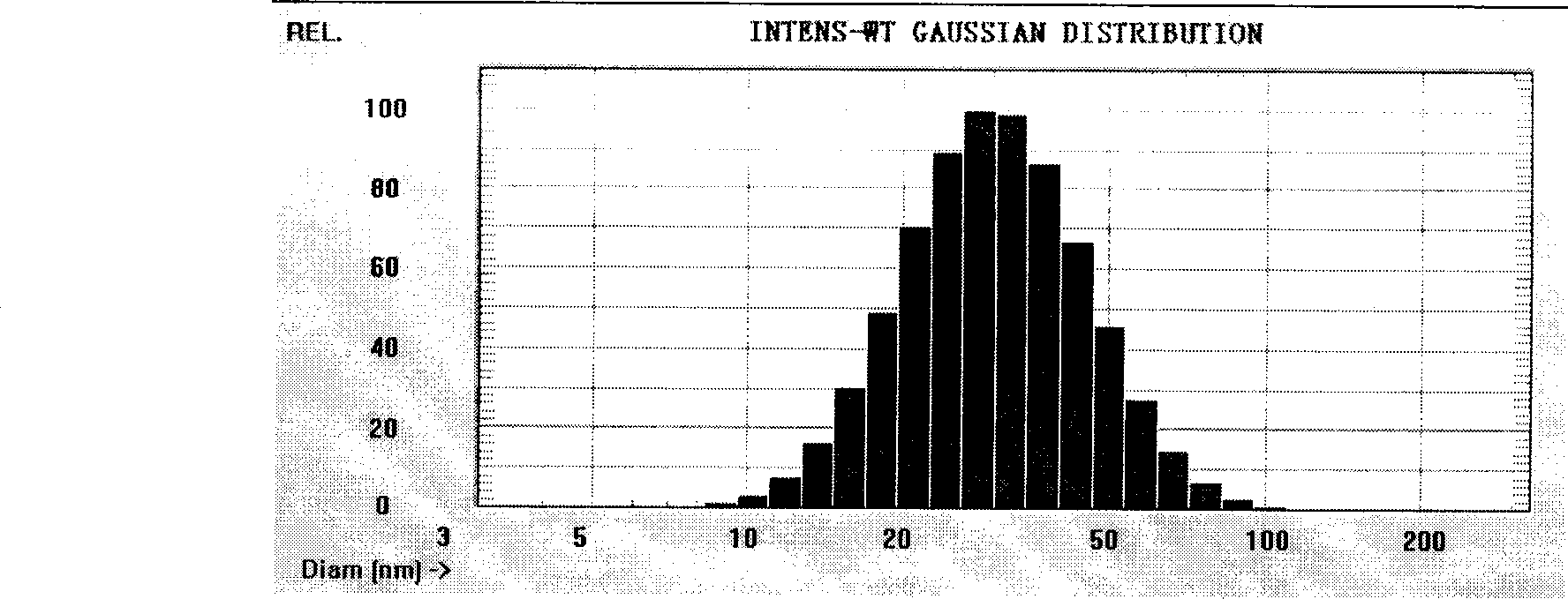
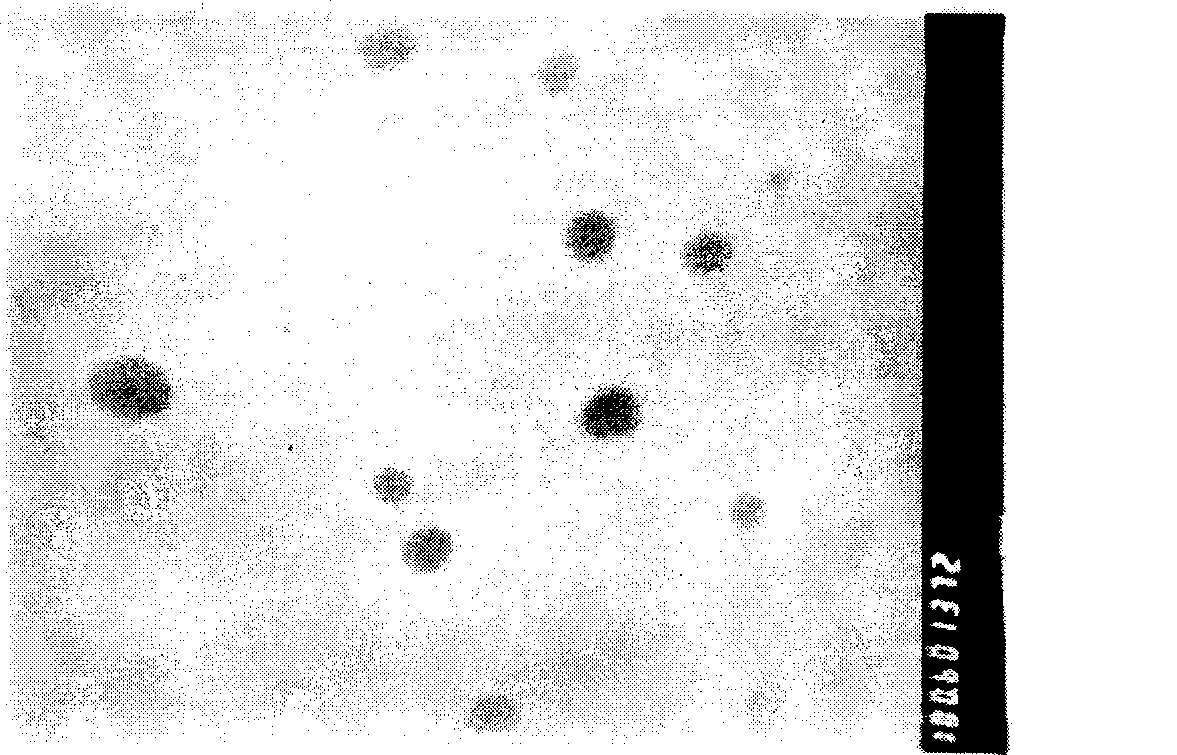
[0033] Dissolve paeonol into isopropyl myristate according to the proportion, then add lecithin and APG to mix and fully dissolve to form the drug-containing inner phase. Add deionized water dropwise to the drug-containing inner phase while stirring at room temperature to Stir at a constant speed of 200r / min for 2h to form a clear and transparent paeonol microemulsion. Measure paeonol microemulsion particle size with Zetasizer Nano ZS90 measuring instrument, see the result figure 1 , the particle size range is 30-50nm, the particle size is uniform, and the appearance is clear, see ...
Embodiment 2
[0034] Example 2. Paeonol microemulsion preparation was prepared by sonication method, and the components and proportioning were the same as in Example 1.
[0035] Dissolve paeonol into isopropyl myristate, then add lecithin and APG to mix and fully dissolve to form the drug-containing inner phase, mix the drug-containing inner phase with deionized water, and finally use a probe ultrasonic instrument at a power of 250w Next, sonicate for 3 minutes to obtain paeonol microemulsion. Zetasizer Nano ZS90 was used to measure the particle size of paeonol microemulsion, the particle size range was 30-50nm, the particle size was uniform, and the appearance was clear.
Embodiment 3
[0036] Example 3. Paeonol microemulsion preparation was prepared by high-pressure homogenization method, and the components and proportioning were the same as in Example 1.
[0037] Dissolve paeonol into isopropyl myristate, then add lecithin and APG to mix and fully dissolve to form the drug-containing inner phase, mix the drug-containing inner phase with deionized water, and use a high-pressure homogenizer in the primary valve 60-70bar, the secondary valve is 600-700bar, homogenize the milk for 3 times to get paeonol microemulsion. Zetasizer Nano ZS90 was used to measure the particle size of paeonol microemulsion, the particle size range was 30-50nm, the particle size was uniform, and the appearance was clear.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com