Preparation and application of insoluble drug-entrapped poloxamer/amphiphilic polysaccharide mixed micelle
An amphiphilic polysaccharide and insoluble drug technology, which is applied in the directions of drug delivery, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the poloxamer micelle encapsulation efficiency and drug loading The problems of low amount, low micellar stability, and high critical micellar concentration can achieve good solubilization, improve stability, and improve oral bioavailability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
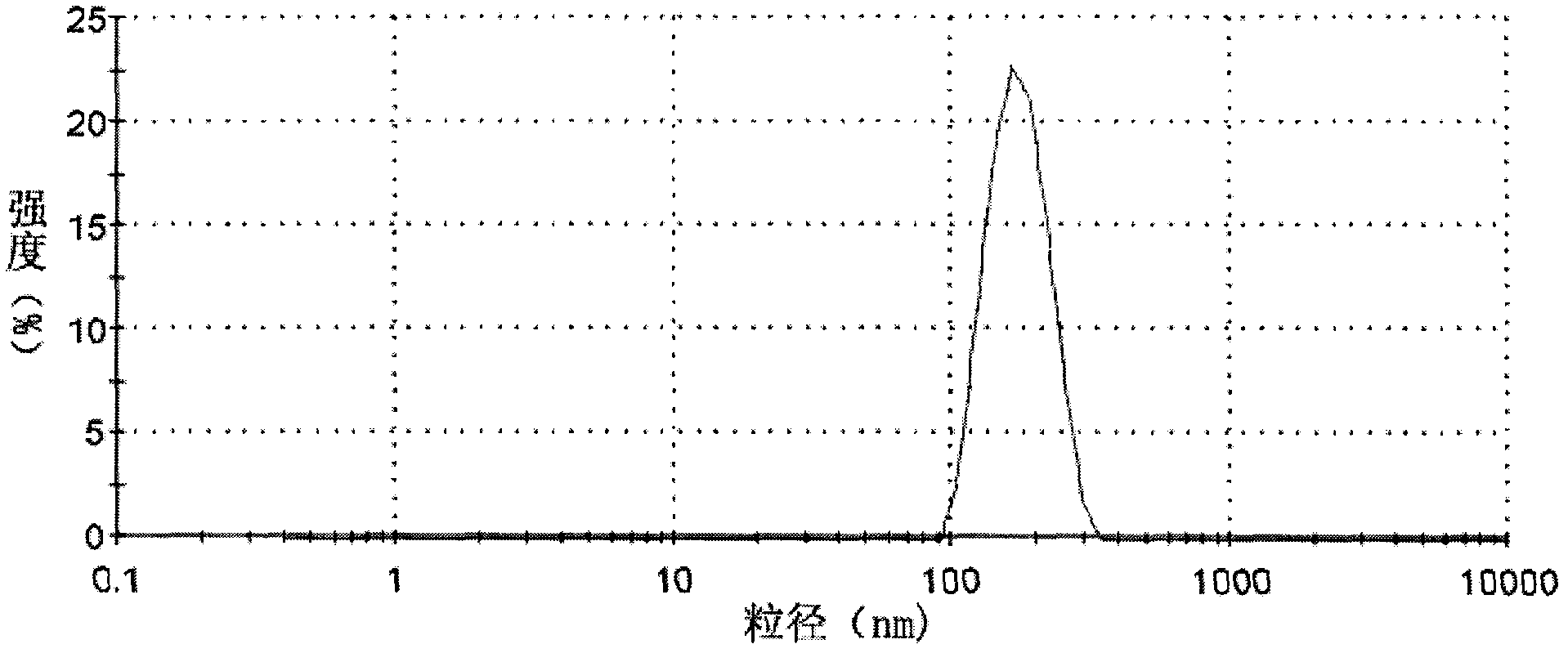
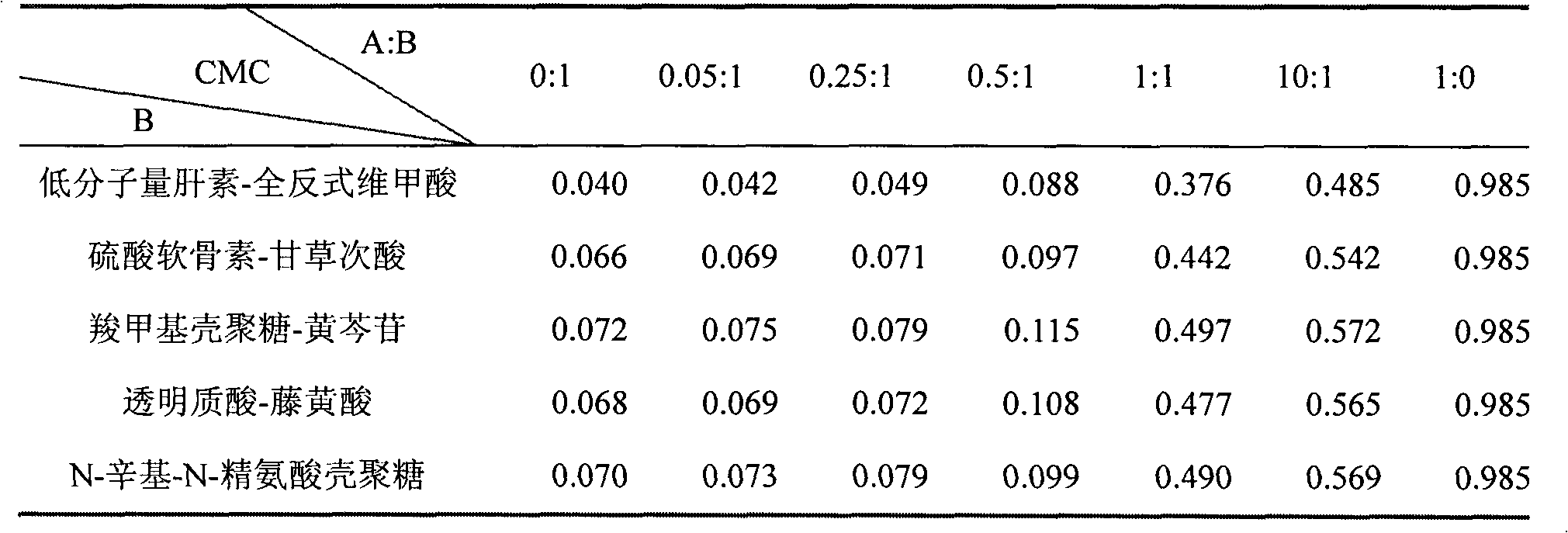
[0024] Example 1: Determination of CMC value of poloxamer / amphipathic polysaccharide conjugate
[0025] 1. Critical micelle concentration (CMC): CMC was determined by fluorescent probe method. Using pyrene as a fluorescent probe, pyrene is a hydrophobic aromatic compound that is extremely sensitive to changes in the polarity of the environment. When the concentration of the poloxamer / amphiphilic polysaccharide conjugate is lower than the CMC, micelles will not form in the solution, and pyrene dissolves in polar water; when the concentration is higher than the CMC, micelles form, and pyrene The hydrophobic part of the micelle core is assigned to enter the non-polar environment, and then a series of changes can be observed in its fluorescence spectrum, such as the enhancement of the fluorescence intensity, the change of the vibration fine structure in the emission spectrum, and the (0,0 ) band redshift. Therefore, by taking the I in the emission spectrum of pyrene 1 / I 3 Rat...
Embodiment 2
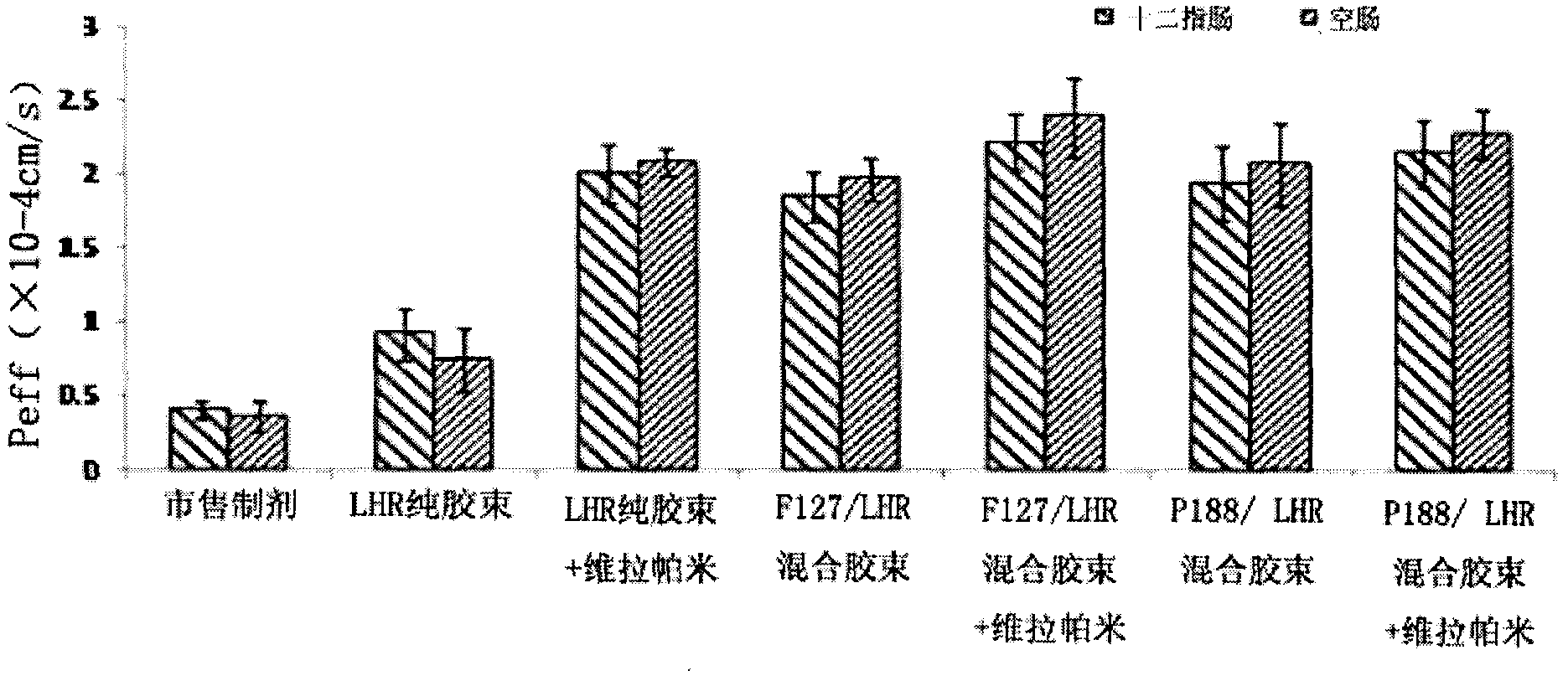
[0032] Example 2: Preparation and characterization of poloxamer / low molecular weight heparin-all-trans retinoic acid mixed micelles loaded with paclitaxel
[0033] 1. Preparation process:
[0034] (1) Dialysis
[0035] 18mg of low molecular weight heparin-all-trans retinoic acid was dissolved in 3ml of distilled water, 20mg of poloxamer was dissolved in 1ml of distilled water, and stirred for 30min respectively. The low molecular weight heparin-all-trans retinoic acid solution was mixed with poloxamer P188 in different weight ratios. Paclitaxel 10mg was dissolved in ethanol. Add paclitaxel / ethanol solution to the mixed solution, stir at room temperature for 15 minutes, sonicate with an ice bath probe for 30 minutes, dialyze in distilled water for 1 day (MWCO=3500), centrifuge the product after dialysis at 3000 rpm for 10 minutes, filter the supernatant with a 0.8 μm filter membrane, and freeze The paclitaxel-loaded poloxamer / low molecular weight heparin-all-trans retinoic aci...
Embodiment 3
[0045] Example 3: Preparation of poloxamer / chondroitin sulfate-glycyrrhetinic acid mixed micelles loaded with itraconazole
[0046] 20mg of chondroitin sulfate-glycyrrhetinic acid was dissolved in 4ml of distilled water, 20mg of poloxamer was dissolved in 1ml of distilled water, and stirred for 30min respectively. Chondroitin sulfate-glycyrrhetinic acid solution was mixed with 250 μL of poloxamer. Itraconazole 10mg was dissolved in ethanol. Add itraconazole / ethanol solution to the mixed solution, stir at room temperature for 15 minutes, sonicate with an ice bath probe for 30 minutes, dialyze with distilled water for 1 day (MWCO=3500), centrifuge the product after dialysis at 3000 rpm for 10 minutes, and filter the supernatant with a 0.8 μm membrane Filter and freeze-dry to obtain the poloxamer / chondroitin sulfate-glycyrrhetinic acid mixed micelles loaded with itraconazole, measure the content of itraconazole in the mixed micelles by HPLC, and calculate according to formula (1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com