Self-assembled nano-system of unsaturated fatty acid-anti-tumor drug conjugates as well as preparation method and application thereof
A technology for unsaturated fatty acids and anti-tumor drugs, which can be used in anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., and can solve problems such as toxic side effects, low drug loading, and drug leakage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
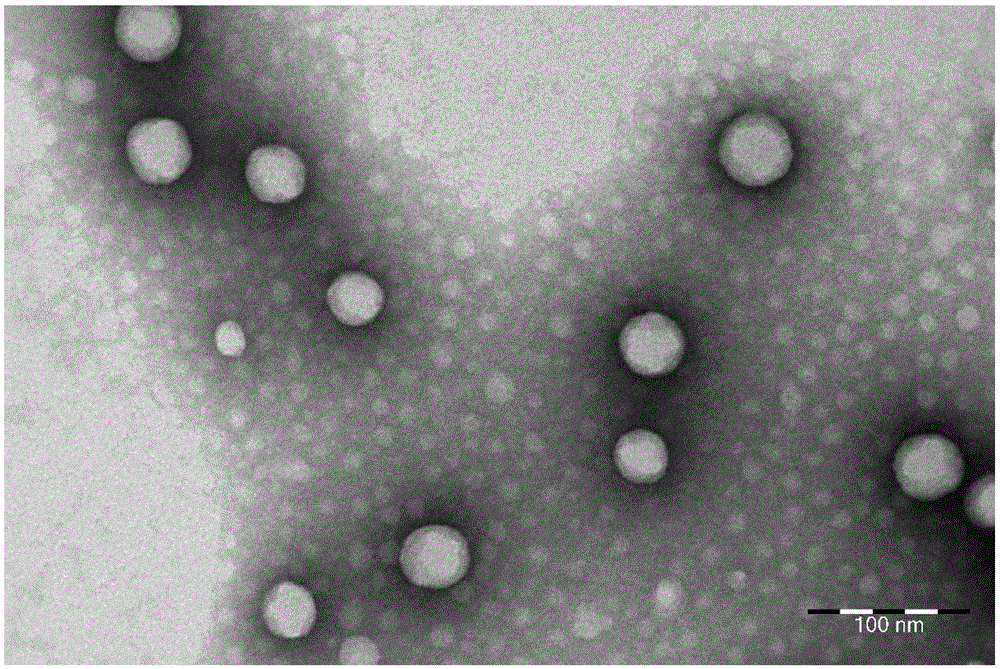
Image
Examples
Embodiment 1
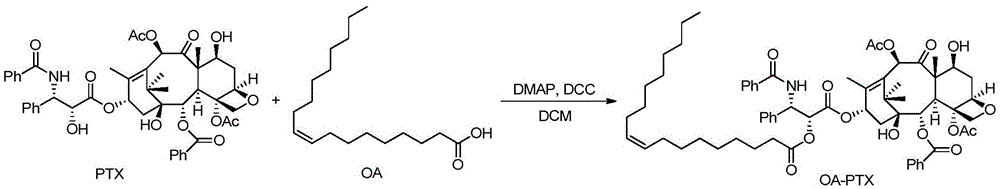
[0102] Embodiment 1: the synthesis of oleic acid-paclitaxel conjugate
[0103] Under nitrogen protection, paclitaxel (85mg, 0.1mmol) was dissolved in an appropriate amount of anhydrous dichloromethane, dimethylaminopyridine (14.4mg, 0.1mmol) and dicyclohexylcarbodiimide (24.6mg, 0.2mmol) were added ), and then added oleic acid (28mg, 0.1mmol) under stirring conditions, and continued to stir and react at room temperature for 12 hours. Remove the precipitate by filtration, dry the filtrate under reduced pressure and vacuum, dissolve it with a small amount of solvent and separate it by thin-layer analysis. The oleic acid-paclitaxel conjugate was obtained (63% yield).
[0104]
Embodiment 2
[0105] Example 2: Synthesis of α-linolenic acid-paclitaxel conjugates
[0106] Under nitrogen protection, paclitaxel (85mg, 0.1mmol) was dissolved in an appropriate amount of anhydrous dichloromethane, dimethylaminopyridine (14.4mg, 0.1mmol) and dicyclohexylcarbodiimide (24.6mg, 0.2mmol) were added ), and then added oleic acid (28mg, 0.1mmol) under stirring conditions, and continued to stir and react at room temperature for 12 hours. Remove the precipitate by filtration, dry the filtrate under reduced pressure and vacuum, dissolve it with a small amount of solvent and separate it by thin-layer analysis. The α-linolenic acid-paclitaxel conjugate was obtained (68% yield).
[0107]
Embodiment 3
[0108] Embodiment 3: the synthesis of DHA-SN38 conjugate
[0109] Under nitrogen protection, 7-ethyl-10-hydroxycamptothecin (40mg, 0.1mmol) was dissolved in an appropriate amount of anhydrous N,N-dimethylformamide, and dimethylaminopyridine (14.4mg, 0.1mmol ) and dicyclohexylcarbodiimide (24.6mg, 0.2mmol), then added conjugated linoleic acid (33mg, 0.1mmol) under stirring conditions, and continued stirring at room temperature for 12 hours. Remove the precipitate by filtration, dry the filtrate under reduced pressure and vacuum, dissolve it with a small amount of solvent and separate it by thin-layer analysis. The DHA-SN38 conjugate was obtained (63% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com