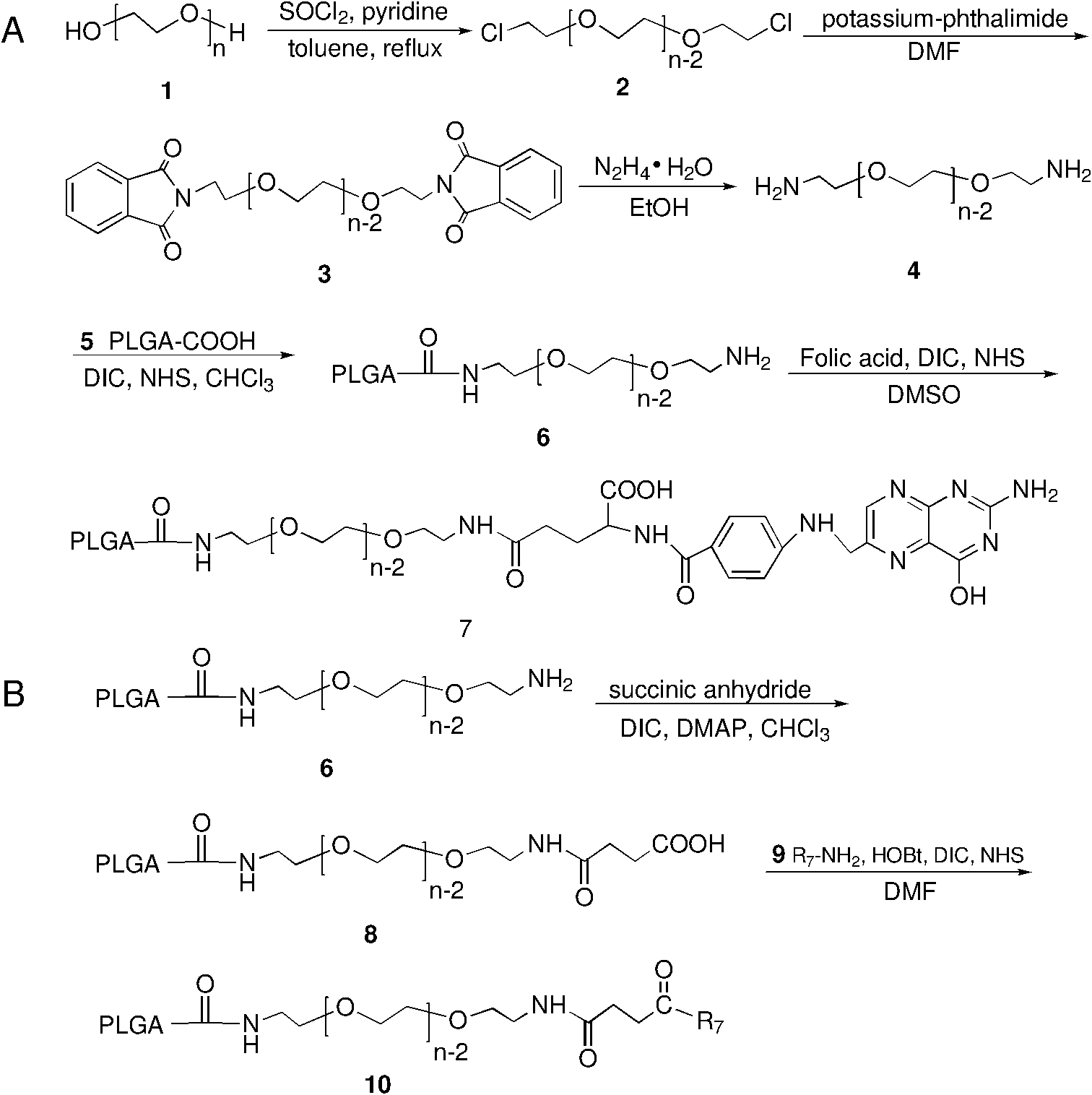Preparation and application methods of difunctional naonparticle preparation entrapping vincristine sulphate
A vincristine sulfate and nanoparticle technology, applied in the field of medicine, can solve the problems of wide particle size distribution, no significant improvement in curative effect, and low targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]Polymers PLGA-PEG-folate and PLGA-PEG-R 7 synthesis, such as figure 1 As shown, it specifically includes the following steps:
[0035] 1) Starting from HO-PEG-OH, through three steps, the polymer NH 2 -PEG-NH 2 ;
[0036] 2) Using chloroform as a solvent, NHS as an activation reagent, and DIC as a condensation agent to make NH 2 -PEG-NH 2 Conjugate with PLGA-COOH to get PLGA-PEG-NH 2 , the above synthesis process see figure 1 A;
[0037] 3) Using chloroform as a solvent, DIC as a condensation agent, and 4-dimethylaminopyridine (DMAP) as a catalyst, succinic anhydride and PLGA-PEG-NH 2 Condensation to obtain PLGA-PEG-succinate;
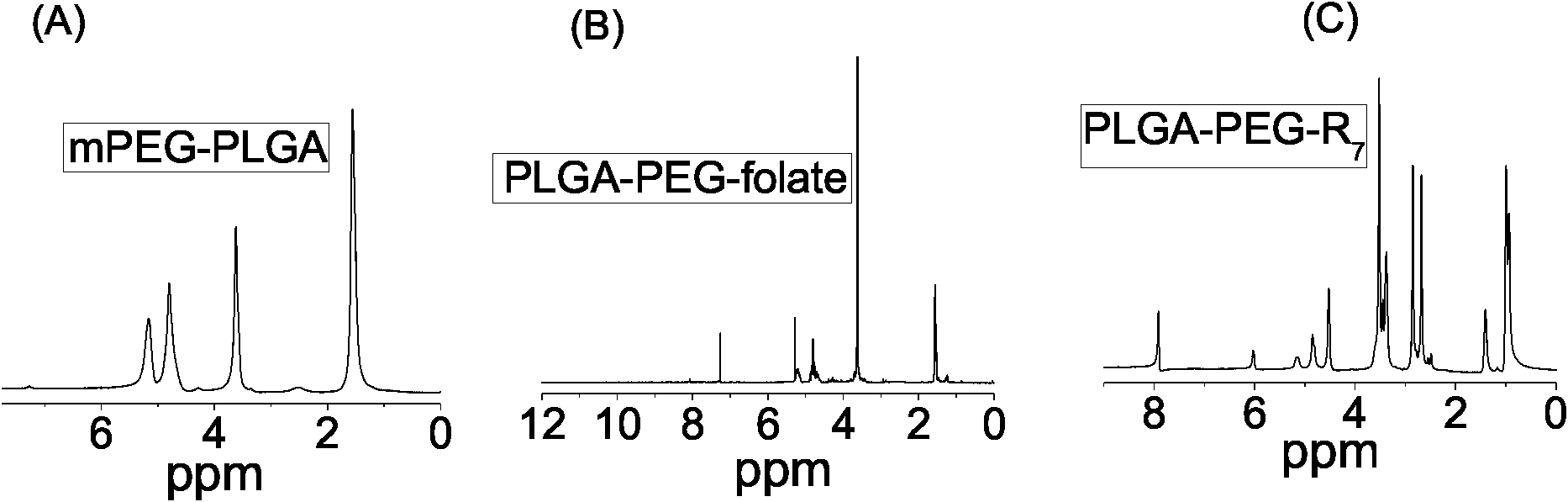
[0038] 4) using DMF as a solvent, the heptameric arginine (R 7 -NH 2 ) reacted with PLGA-PEG-succinate to obtain cell-penetrating peptide modified PLGA-PEG (PLGA-PEG-R 7 ); 1 See HNMR figure 2 c. In the second step reaction, the molar ratio of PLGA-COOH / NHS / DIC is: 1 / 5 / 5~1 / 10 / 10. 3) and 4) step synthesis process see figure 1 b. ...
Embodiment 2
[0041] The preparation of the PLGA-PEG bifunctional nanoparticles modified by folic acid / cell penetrating peptide comprises the following steps:
[0042] (1) Dissolve vincristine sulfate in Tris-HCl buffer solution with pH (5-7.4) to form a concentrated solution (referred to as phase I). The three polymer carriers PLGA-mPEG, PLGA-PEG-folate and PLGA-PEG-R 7 (mass ratio 7 / 2 / 1) dissolved in an organic solvent (dichloromethane, or chloroform, or ethyl acetate) (referred to as phase II). The ratio of the buffer solution to the organic solvent is 1 / 10-1 / 30. Theoretically, the dosage of vincristine sulfate is 4-9% (w / w);
[0043] (2) Ultrasound, under the condition of ice-water bath, add phase I dropwise to phase II to obtain colostrum;
[0044] (3) Ultrasound, under the condition of ice-water bath, add the above-mentioned colostrum dropwise to the Tris-HCl buffer solution containing 0.6-2.5% PVA (w / v), pH 5-7.4, to obtain w / o / w double emulsion ;
[0045] (4) stirring at room t...
Embodiment 3
[0052] Release of folate / cell-penetrating peptide-modified PLGA-PEG bifunctional nanoparticles
[0053] Take 0.5mL of the bifunctional nanoparticle solution, put it in a dialysis bag (5000-10000Da), put it in a release medium (phosphate buffer or Tris-HCl buffer) with a volume of 30mL, shake it on a constant temperature shaker at 37°C, and use it for regular sampling. The content of VCR in the release medium was determined by high performance liquid chromatography, and the cumulative release percentage was calculated. see release curve Figure 4 .
[0054] Through the study of the release properties of the samples in two release media, the results showed that, in the first 8 hours, VCR appeared burst release in phosphate buffer (pH 6.8); in 8-20 hours, VCR showed a steady release; but after more than 20 hours, due to VCR has poor stability in phosphate buffered saline, but the concentration decreases slowly, and the cumulative release decreases accordingly ( Figure 4 A). ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com