Targeted polymeric prodrugs containing multifunctional linkers
A linker and water-soluble polymer technology, applied in the direction of carbohydrate active ingredients, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of anticancer drug toxicity and achieve the effect of enhancing bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0094] Preferred embodiments include:
[0095]
[0096]
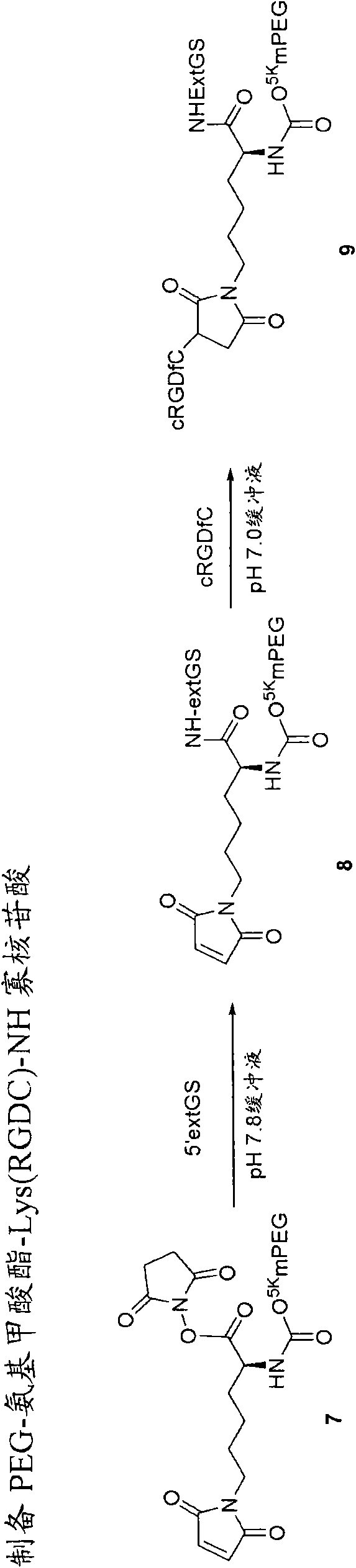
[0097] C. Substantially non-antigenic water-soluble polymers (R 1 )
[0098] The prodrugs of the present invention include polymer residues R 1 , preferably a water-soluble and substantially non-antigenic polymer. Suitable examples of such polymers include polyalkylene oxides (PAOs) such as polyethylene glycols. Some preferred polymers are polyethylene glycols such as mPEG. Thus, non-limiting examples of such polymers include polyalkylene oxide homopolymers such as polyethylene glycol (PEG) or polypropylene glycol, polyoxyethylenated polyols, copolymers thereof, and block copolymers thereof.
[0099] The polymer portion of the compounds described herein has an average molecular weight of from about 2,000 to about 100,000 Daltons, preferably from about 5,000 to about 60,000 Daltons. In some aspects, the polyalkylene oxide may be from about 5,000 to about 25,000, preferably from about 12,000 to about 20,000 dal...
Embodiment 1
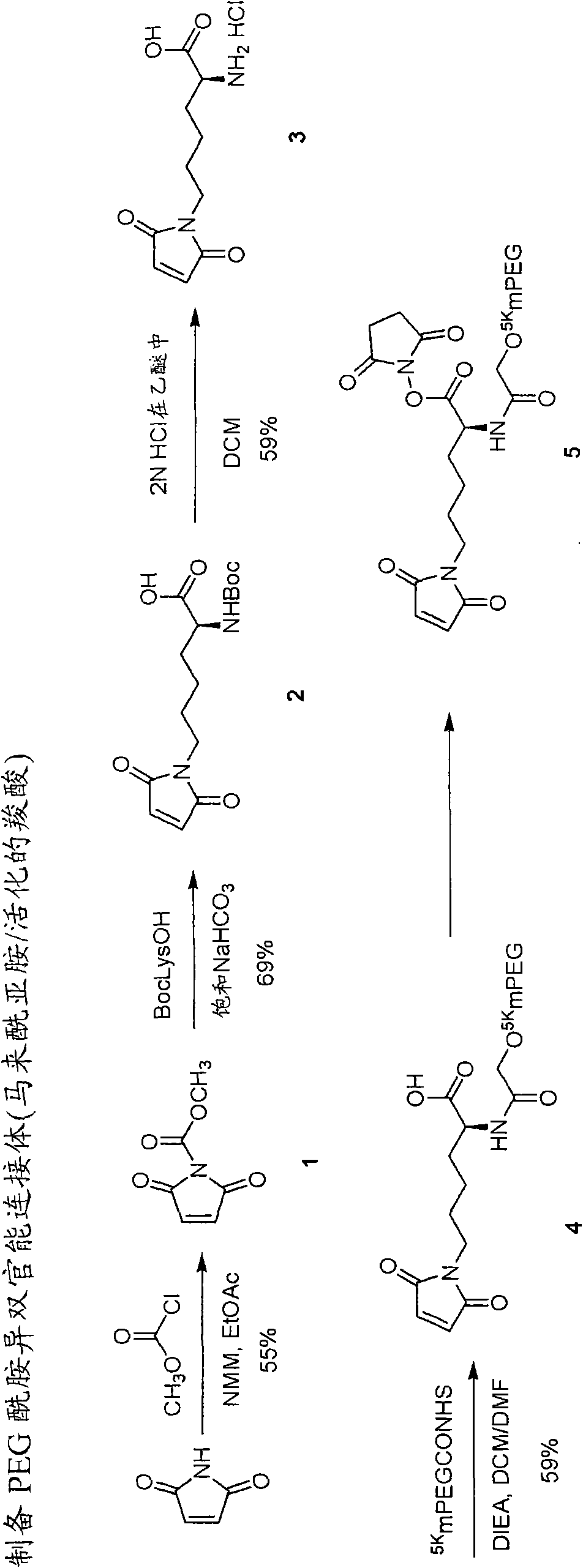
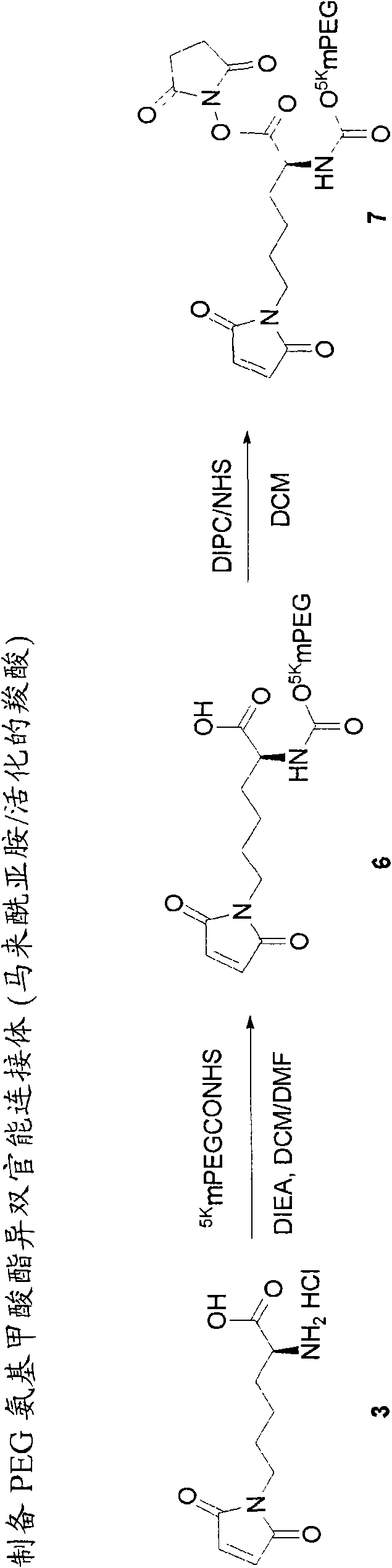
[0483] Example 1: N-(methoxycarbonyl)maleimide (Compound 1).
[0484] Add methyl chloroformate (4.4 mL, 56.7 mmol, 1 eq) to maleimide (5.5 g, 56.7 mmol, 1 eq) and NMM (6.2 mL, 56.7 mmol, 1 eq) in 200 mL at 0 °C solution in EtOAc. The suspension was stirred at 0 °C for 30 min, filtered and washed with EtOAc. The filtrate and washings were combined, washed with cold water, and washed with anhydrous Na 2 SO 4 dry. After filtration and evaporation in vacuo, a pink solid was obtained. Purification by silica gel column chromatography (hexane-EtOAc, 1:1, v / v) afforded the product (4.8 g, 55%).
Embodiment 2
[0485] Example 2: Nε-maleoyl-α-(Boc)-L-lysine (Compound 2).
[0486] Add N-(methoxycarbonyl)maleimide (315 mg, 2.03 mmol, 1 equiv) to Boc-L-lysine (500 mg, 2.03 mmol, 1 equiv) in 10 mL of saturated NaHCO at 0 °C 3 in solution in aqueous solution. The mixture was stirred vigorously at 0°C for 40 minutes and at room temperature for a further 1 hour. After cooling to 0°C, use concentrated H 2 SO 4 The solution was acidified to pH 3.0, then extracted with ethyl acetate. The organic layers were combined and washed with anhydrous MgSO 4 dry. After removing the solvent in vacuo, the residue was purified by silica gel column chromatography using CHCl 3 - MeOH mixture (5:1, v / v) to give the product (458 mg, 69%): 1 H NMRδ1.39-1.87 (6H, m, (CH 2 ) 3 ), 1.44 (9H, s, Boc), 3.52 (2H, t, J=7.2Hz, NCH 2 ), 4.25-4.30 (1H, m, CH), 5.15 (1H, d, NH), 6.70 (2H, s, maleimide).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average molecular weight | aaaaa | aaaaa |
| Average molecular weight | aaaaa | aaaaa |
| Average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com